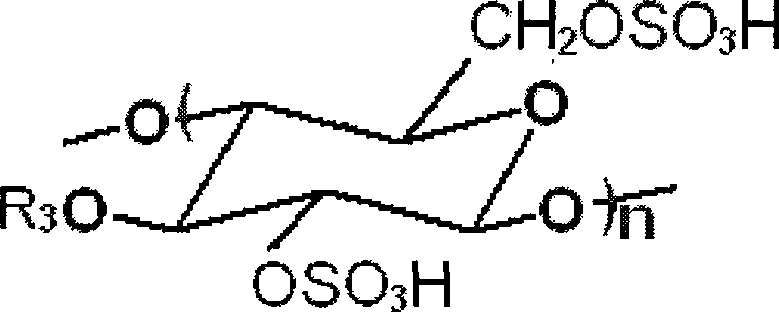
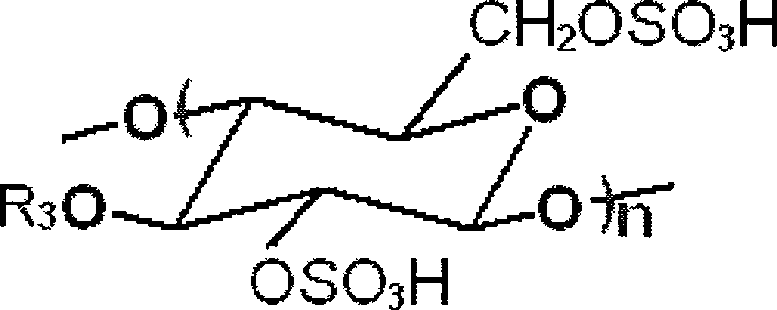
Preparation method of beta-1,4-glucan-6,2,3-sulfate
A sulfate and dextran technology, applied in the field of β-1, can solve problems such as complicated steps, non-compliance, and no mention, and achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of cellulose ionic liquid homogeneous system: add microcrystalline cellulose to ionic liquid 1-butyl-3-methylimidazolium chloride ([Bmim]Cl), in 100°C oil bath at 300r / min Stir at a high speed for 12 hours to dissolve it, and obtain a cellulose ionic liquid homogeneous system;
[0035] (2) Sulfation: Add concentrated sulfuric acid to the cellulose ionic liquid homogeneous system obtained in step (1), the amount of concentrated sulfuric acid is 3.0mol / molAGU (anhydroglucose unit in cellulose); at 35°C, the stirring rate is 800r After carrying out the sulfation reaction under the condition of 1 / min for 8 hours, add 4%, the sodium acetate-ethanol solution of w / v, its consumption is 2 times of reactant volume; The product is precipitated out, and the product is filtered, washed to obtain Crude product of esterification;
[0036] (3) Degradation: the crude product of the esterification product is dissolved in NaH with a pH value of 7.5 2 (PO 4 ) 3 -Na 2 ...
Embodiment 2
[0039] (1) Preparation of cellulose ionic liquid homogeneous system: Add ordinary cellulose to ionic liquid 1-allyl-3-methylimidazole chloride ([Amim]Cl), and heat it in an oil bath at 100°C at 300r / min Stir at a high speed for 12 hours to dissolve it, and obtain a cellulose ionic liquid homogeneous system;
[0040] (2) Sulfation: Add chlorosulfonic acid to the cellulose ionic liquid homogeneous system obtained in step (1), and the consumption of chlorosulfonic acid is 4.5mol / molAGU (anhydroglucose unit in cellulose); After carrying out the sulfation reaction under the condition of 1000r / min for 6 hours, add 4%, the sodium acetate-ethanol solution of w / v, its consumption is 4 times of reactant volume; The product is precipitated out, and the product is filtered, Washing obtains the crude product of the esterification product;
[0041] (3) Degradation: the crude product of the esterification product is dissolved in NaH with a pH value of 9.0 2 (PO 4 ) 3 -Na 2 H(PO 4 ) 3 In...
Embodiment 3
[0044] (1) Preparation of cellulose ionic liquid homogeneous system: add commercially available microcrystalline cellulose to ionic liquid 1-butyl-3-methylimidazole chloride ([Bmim]Cl), Stir at a rate of 300r / min for 12 hours to dissolve it, and obtain a homogeneous cellulose ionic liquid system;
[0045] (2) Sulfation: Add sulfur trioxide to the cellulose ionic liquid homogeneous system obtained in step (1), and the amount of sulfur trioxide is 4.0mol / molAGU (anhydroglucose unit in cellulose); Carry out sulfation reaction under the condition of 900r / min after 7 hours, add 4%, w / v sodium acetate-ethanol solution, its dosage is 3 times of reaction volume; The product is precipitated out, and the product is filtered and washed Obtain the crude product of esterification product;
[0046] (3) Degradation: Dissolve the crude product of the esterification product in NaH with a pH value of 8.0 2 (PO 4 ) 3 -Na 2 H(PO 4 ) 3 In the buffer solution, then add endocellulase 1 (EGL) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com