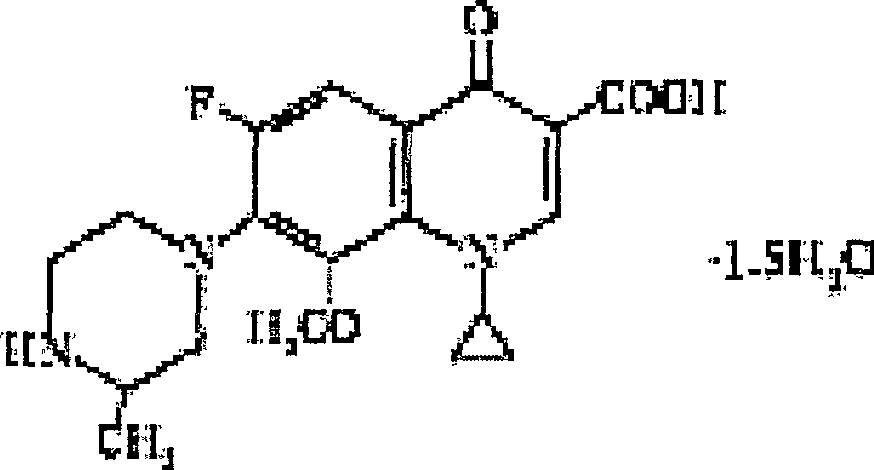
Gatifloxacin freeze-dried powder injection and preparing method thereof
A technology of freeze-dried powder injection and gatifloxacin, which is applied in the field of medicine, and can solve the problem of poor clarity and stability of freeze-dried powder injection of gatifloxacin, poor clarity of freeze-dried powder injection, and low content of main ingredients, etc. problems, to achieve the effect of good appearance, solve the problem of dissolution, and low water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Formula composition: prescription (1000 pieces)
[0057] Gatifloxacin 200.0g
[0058] Mannitol 40.0g
[0059] Add water for injection to 3000ml
[0060] 2. Preparation process
[0061] Weigh the formulated amount of gatifloxacin and mannitol into a sterile container, add an appropriate amount of water for injection at 70°C, dissolve, keep warm and stir for 30 minutes, then adjust the pH value to 4.0 with 0.05mol / L lactic acid solution, and stir well; Use charcoal (0.1%), keep warm and stir at 80°C for 15 minutes, let it stand, filter and decarbonize, take the filtrate, then dilute the filtrate to 3000mL with water for injection, stir evenly, measure the intermediate, pass 0.45μm, 0.22μm composite microporous membrane filter, aseptically put in 10ml vials, 3ml per bottle, cover the bottle and keep the air outlet, and then freeze-dry: first lower the drug from room temperature to -18.6°C, and keep it warm for 20 minutes Finally, pre-freeze at -40°C for 3 hours; the...
Embodiment 2
[0063] 1. Prescription composition: prescription (1000 pieces)
[0064] Gatifloxacin 400.0g
[0065] Mannitol 80.0g
[0066] Add water for injection to 6000ml
[0067] 2. Preparation process
[0068] Weigh the formulated amount of gatifloxacin and mannitol into a sterile container, add an appropriate amount of water for injection at 80°C, dissolve, keep warm and stir for 15 minutes, then adjust the pH value to 4.5 with 0.15mol / L lactic acid solution, and stir well; Use charcoal (0.1%), heat and stir at 60°C for 20 minutes, filter and decarbonize, take the filtrate, then dilute the filtrate with water for injection to 6000mL, stir well, measure the intermediate, pass the 0.45μm, 0.22μm composite micro Pore membrane filtration, aseptic filling in 10ml vials, 3ml per bottle, stopper and keep the air outlet, and then freeze-dry: first lower the medicine from room temperature to -18.6°C, keep warm for 40 minutes, continue Cool down to -40°C and pre-freeze for 4 hours, then he...
Embodiment 3
[0070] 1. Prescription composition
[0071] Gatifloxacin 100.0g
[0072] Mannitol 20.0g
[0073] Add water for injection to 1500ml
[0074] 2. Preparation process
[0075] Weigh gatifloxacin and mannitol into a sterile container, add an appropriate amount of water for injection at 75°C, dissolve, stir, adjust the pH value to 3.5 with 0.15mol / L lactic acid solution, and stir well; add charcoal for needles (0.1%), Insulate and stir at 70°C for 30 minutes, filter and decarbonize, take the filtrate, then dilute it with water for injection to 1500mL, stir evenly, measure the intermediate, pass through 0.45μm, 0.22μm composite microporous filter membrane after passing, sterile Fill in 10ml vials, 3ml per bottle, cover the cork and keep the air outlet, and then freeze-dry: first lower the drug from room temperature to -18.6°C, keep it warm for 30 minutes, and then continue to cool down to -40°C Pre-freeze for 2 hours under the same conditions, then heat the drug from -40°C to -23...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com