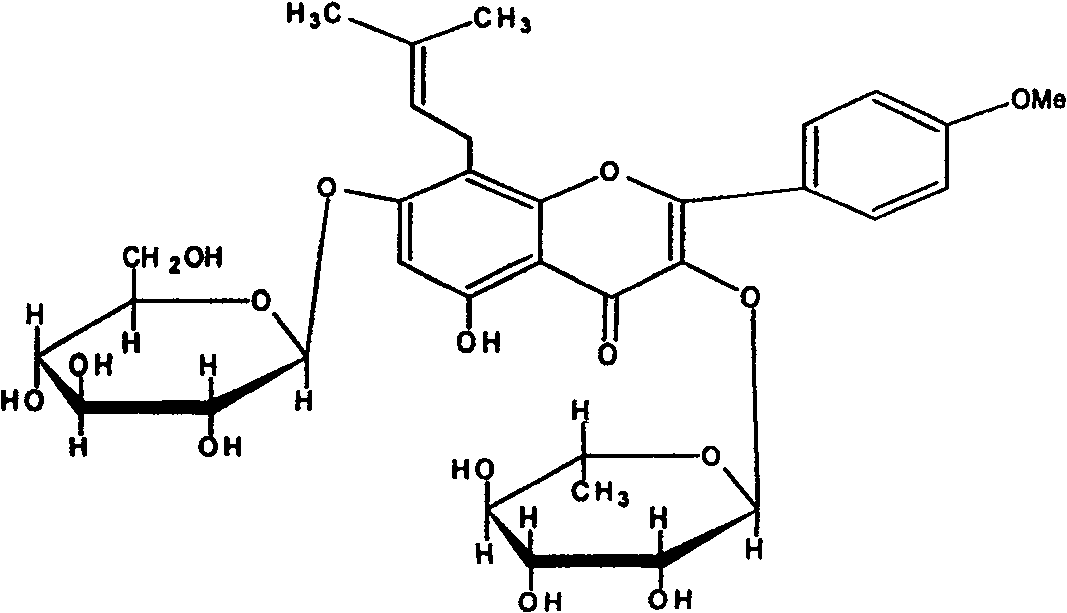
Novel medicinal uses of icariin
A technology of icariin and medicine, applied in the field of pharmaceutical use of icariin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0012] Preparation Example 1: Preparation of Icariin Injection
[0013] Weigh 1.00g of icariin, add 20ml of n-butanol and 30ml of propylene glycol to completely dissolve the saponin, add 0.15g of tetracaine hydrochloride and 0.02g of citric acid into 40ml of water for injection to dissolve completely, and mix the above aqueous solution Add to the drug solution and mix well; adjust the pH value to 5.5±1.0 with borax and boric acid; add 0.1% activated carbon for needles and keep warm at 85°C for 30 minutes, G 3 Filtrate through a vertically fused glass funnel, and filter through a 0.22um microporous membrane; the filtrate is divided into 7ml vials, each containing 2ml. Or lyophilized by a lyophilizer to make a lyophilized powder.
preparation example 2
[0014] Preparation Example 2: Preparation of Icariin Emulsion for Injection
[0015] Icariin
[0016] Dissolve icariin, soy lecithin and cholesterol in ethanol and add them to refined soybean oil, mix until clear, and remove the alcohol by rotary evaporation or nitrogen evaporation to obtain icariin 10mg / ml and 50mg / ml Cholesterol in soybean oil solution. Disperse poloxamer and glycerin into water for injection, slowly add icariin-containing phospholipid and cholesterol oil solution dropwise into the dispersion system under high-speed stirring conditions to prepare a coarse emulsion. The obtained coarse emulsion was further emulsified by means of a homogenizer under a pressure of 100 MPa. After the emulsification was completed, it was sterilized at 115°C for 30 minutes, and then packaged.
experiment example 1
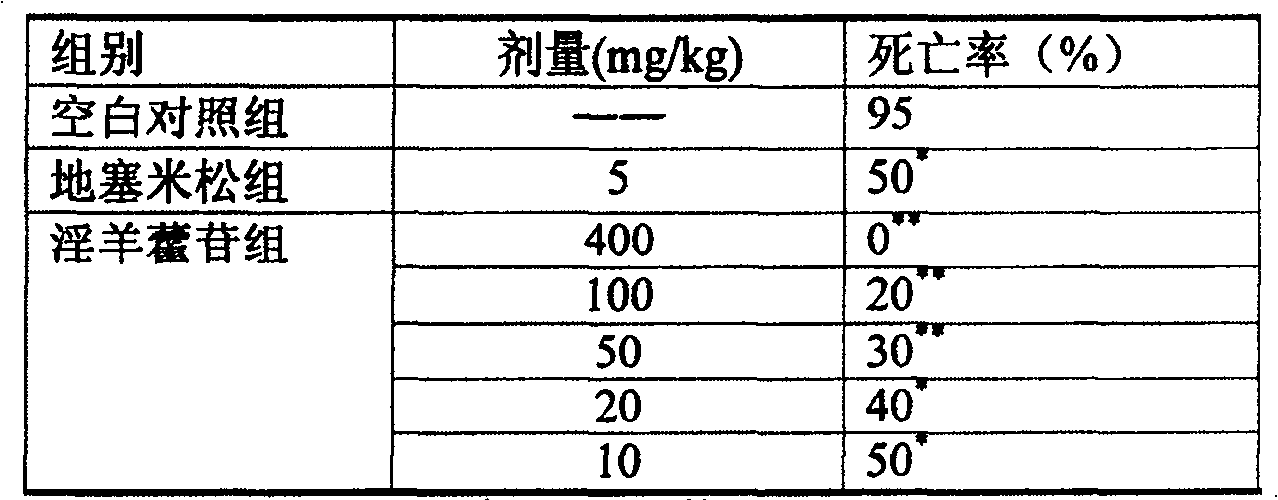
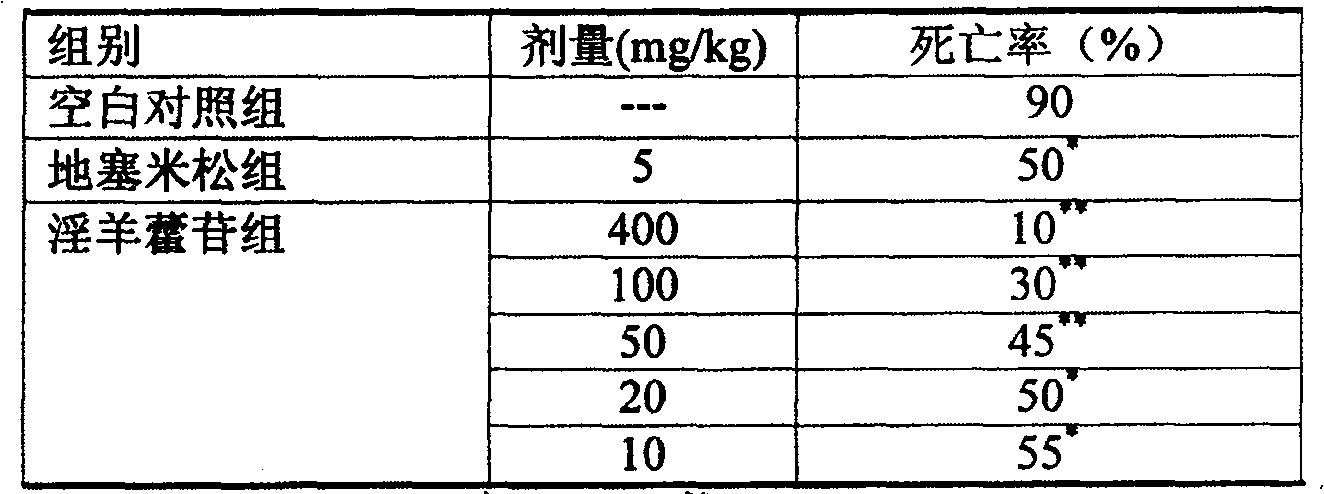
[0017] Experimental example 1 Effect test of icariin on endotoxin lethality in mice
[0018] 1 material
[0019] Icariin injection: prepared according to Preparation Example 1.
[0020] Endotoxin: Department of Microbiology, Shanghai Second Military Medical University, batch number: 020412.
[0021] Second-class Kunming mice, weighing 19-21 g, were provided by the Experimental Animal Center of Shandong Provincial Natural Medicine Engineering Technology Research Center, certificate number: SYXK (Lu) 20030020.
[0022] Dexamethasone sodium phosphate injection: Tianjin Pharmaceutical Lianshui Co., Ltd., specification: 5mg, batch number: 030825.
[0023] 2 Methods and results
[0024] Mice were randomly divided into blank control group, dexamethasone group, icariin 400mg / kg group, 100mg / kg group, 50mg / kg group, 20mg / kg group, 10mg / kg group, 20 in each group, male and female Half. After intravenous injection of endotoxin 20mg / kg, the mice were administered intravenously, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com