Hyperosteogeny-resisting formulation for treating orthopaedics disease, preparation method and quality control method thereof
A technology for anti-bone hyperplasia and orthopedic diseases. It is applied in bone diseases, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve the problems of increasing stability, poorly soluble components, and poor bioavailability, so as to achieve increased stability. , Improve bioavailability and high pass rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1 of the present invention: Rehmannia glutinosa 31.8g, steamed Cistanche 21.3g, salted dog spine 21.3g, salted Ligustrum lucidum 10.5g, epimedium 21.3g, Caulis Spatholobus 21.3g, fried radish seed 10.5g, Drynaria 21.3g, Achyranthes bidentata 21.3g
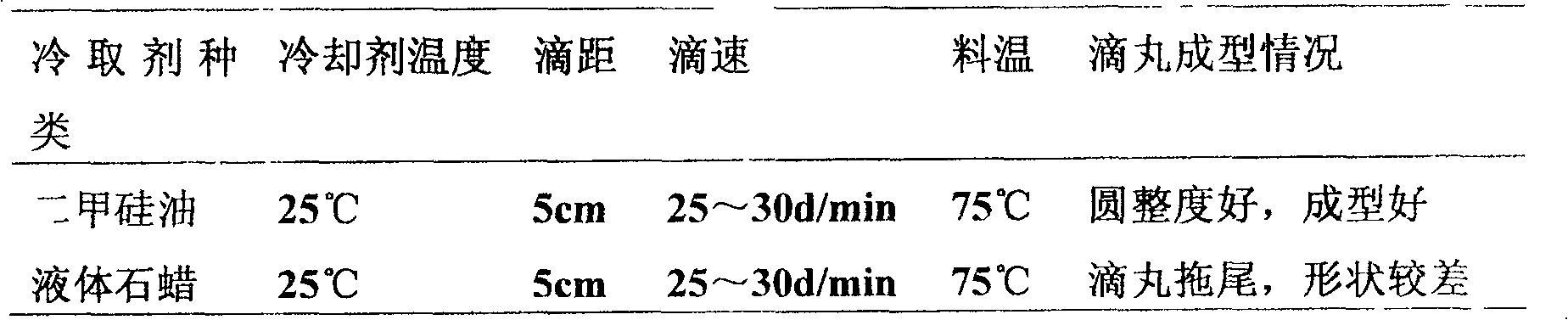
[0112] Take rehmannia glutinosa, steamed cistanche, salted dog spines, salted privet privet seeds, epimedium, Caulis Spatholobus, fried radish seeds, rhizoma drynariae, and achyranthes bidentata, add water and boil twice, the first time for 2 hours, and the second time Second time for 1.5 hours, combine the decoction, filter, concentrate the filtrate to a thick paste with a relative density of 1.30 at 50°C, dry, pulverize, add polyethylene glycol 4000 according to extract powder: matrix = 1:1.5, mix evenly, and heat to 80-90°C, after it is completely melted, transfer it to a liquid storage bottle, keep warm at 70-75°C, drop into simethicone oil at 20-30°C, the drop distance is 4-6cm, and the drop rate is 25-30 drops / ...
Embodiment 2
[0113] Example 2 of the present invention: Rehmannia glutinosa 31.8g, steamed cistanche 21.3g, salted dog ridge 21.3g, salted privet privet 10.5g, epimedium 21.3g, milletanus 21.3g, fried radish seed 10.5g, Drynaria 21.3g, Achyranthes bidentata 21.3g
[0114] Take rehmannia glutinosa, steamed cistanche, salted dog spines, salted privet privet seeds, epimedium, Caulis Spatholobus, fried radish seeds, rhizoma drynariae, and achyranthes bidentata, add water and boil twice, the first time for 2 hours, and the second time 1.5 hours for the second time, combine the decoction, filter, concentrate the filtrate to a thick paste with a relative density of 1.30 at 50°C, dry, pulverize, add 8% cross-linked polyvinylpyrrolidone, 10% microcrystalline cellulose, mix well, and add the concentration 25% ethanol, passed through a 24-mesh sieve for granulation, dried at 60°C for 2 hours, passed through a 24-mesh sieve for granulation, and then mixed with 0.2% magnesium stearate to obtain a table...
Embodiment 3
[0115] Example 3 of the present invention: Rehmannia glutinosa 31.8g, steamed cistanche 21.3g, salted dog ridge 21.3g, salted privet privet 10.5g, epimedium 21.3g, milletanus 21.3g, fried radish seed 10.5g, Drynaria 21.3g, Achyranthes bidentata 21.3g
[0116] Take rehmannia glutinosa, steamed cistanche, salted dog spines, salted privet privet seeds, epimedium, Caulis Spatholobus, fried radish seeds, rhizoma drynariae, and achyranthes bidentata, add water and boil twice, the first time for 2 hours, and the second time The second time is 1.5 hours, the decoction is combined, filtered, the filtrate is concentrated to a thick paste with a relative density of 1.30 at 50°C, dried, crushed, and citric acid with a ratio of 1:2 to the main drug is added, mixed evenly, and anhydrous Use ethanol as a wetting agent to make soft materials, pass through a 24-mesh sieve to obtain wet granules, then put them into a semi-automatic coating granulator with a rotation speed of 80-100r / min, prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com