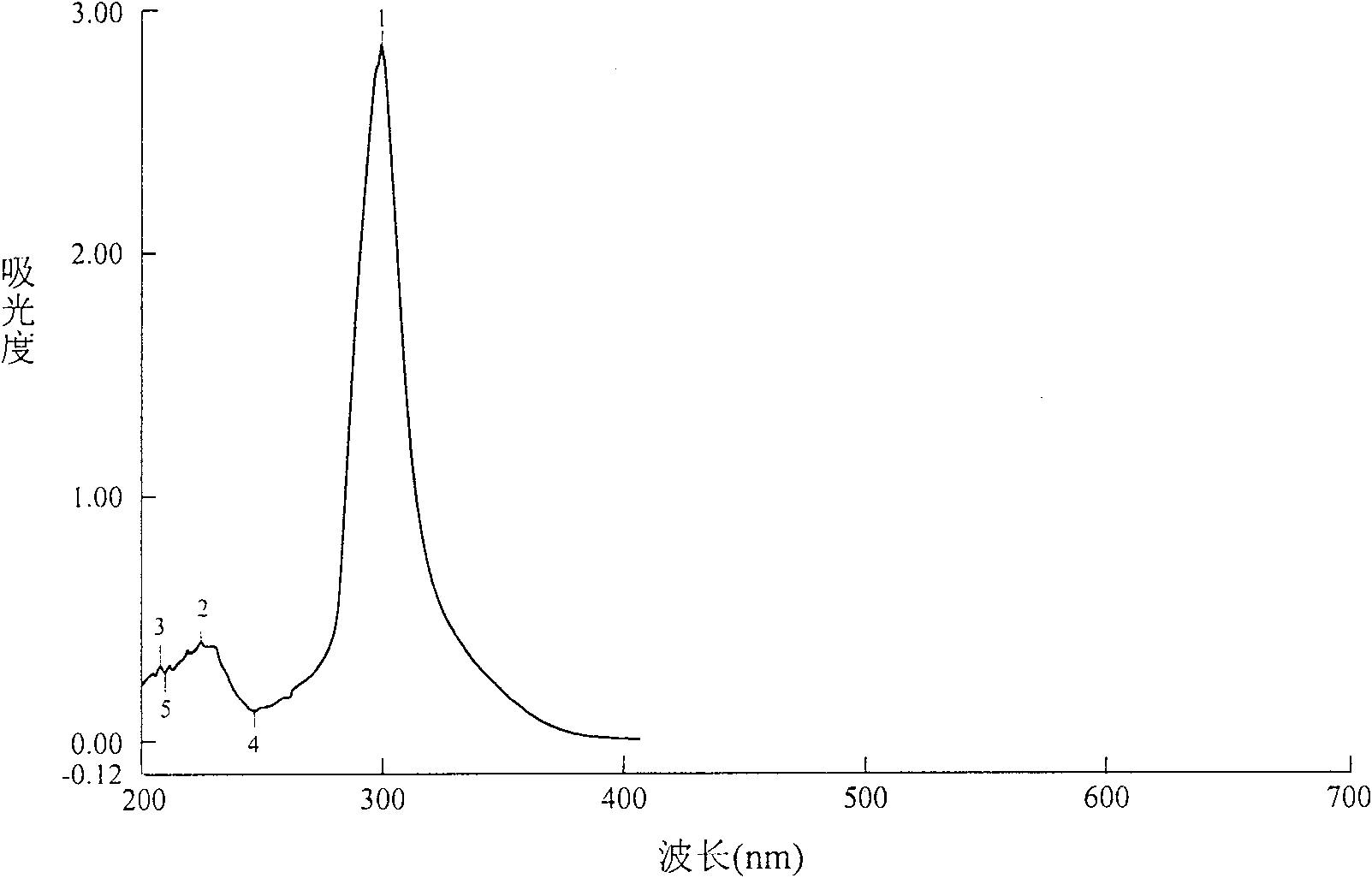
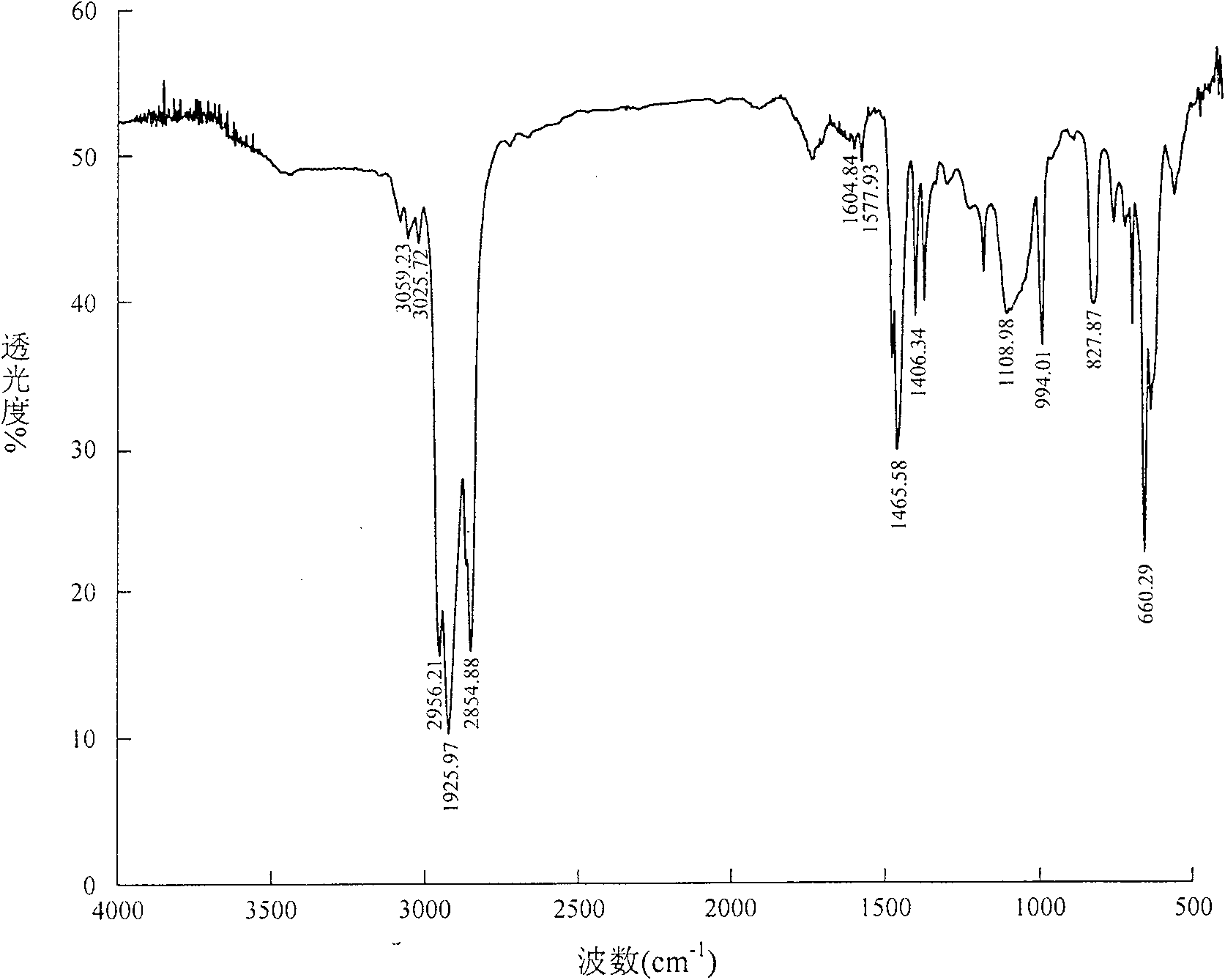
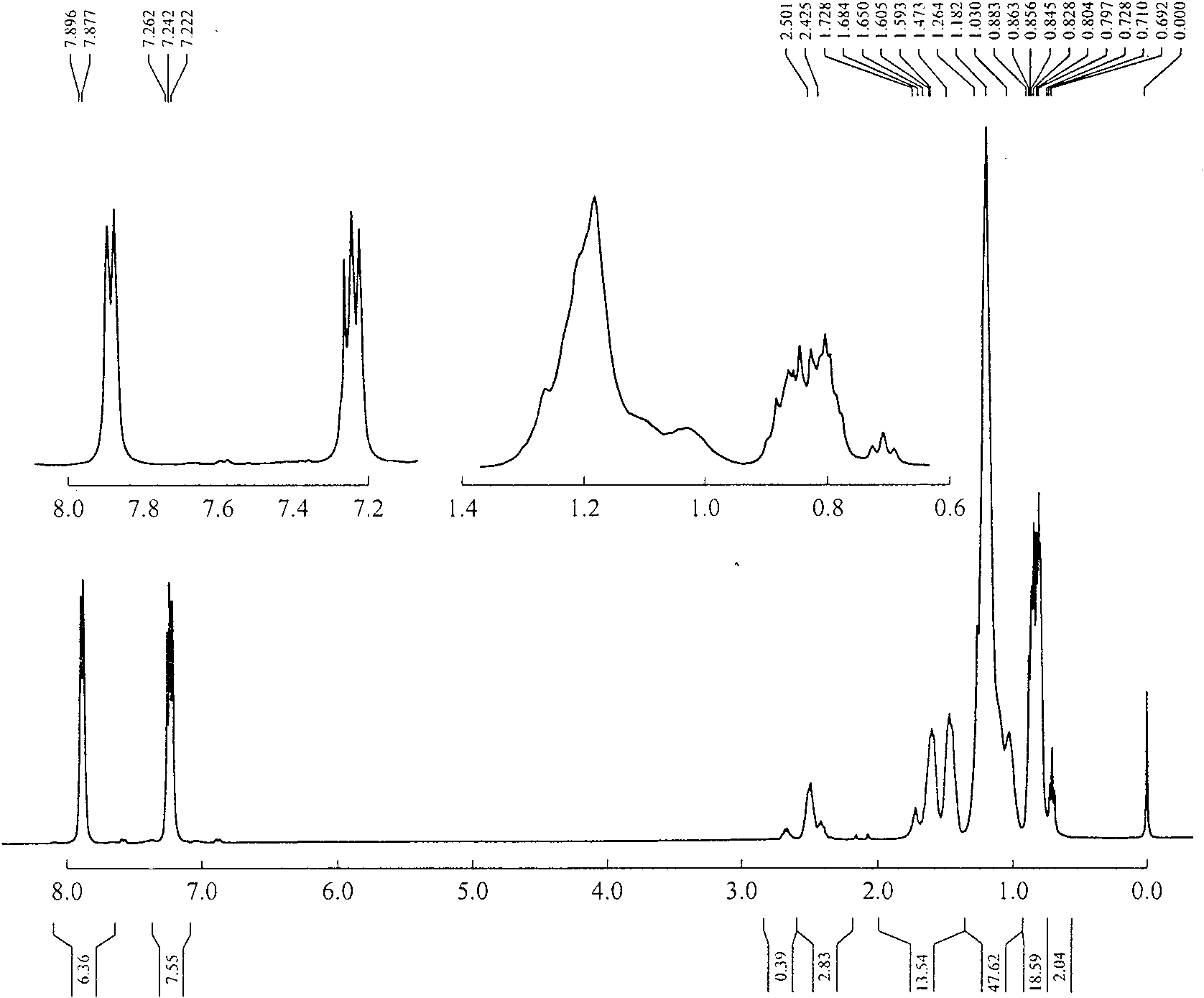
Method for synthesizing 4,4-didetergent alkylate iodonium hexafluoro antimonate
A technology of didodecylbenzene and hexafluoroantimonate, which can be used in antimony organic compounds, chemical instruments and methods, preparation of halogenated hydrocarbons, etc., can solve problems such as toxic cations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] 1. Preparation of 4,4'-Didodecylphenyliodonium Hydrochloride
[0031] Take 20mL of dodecylbenzene and 20mL of acetic anhydride into a 250mL three-necked reaction flask equipped with a thermometer and an electric stirrer, cool to -5°C at low temperature, then add 20g of potassium iodate, and add 14mL dropwise under stirring. For the mixture of 98% concentrated sulfuric acid and 20mL acetic anhydride, the rate of addition should be kept at a temperature not exceeding -5°C. After the dropwise addition, the reaction temperature rises to room temperature, and the reaction is continued for 8 hours, and then 40mL is added dropwise at a temperature lower than 5°C. Distilled water, standing for stratification, extracting the water layer twice with 20mL toluene, combining the organic layers, adding 10g of barium chloride aqueous solution to the combined toluene solution, a large amount of white precipitates precipitated, filtered, washed with water, and dried the filtrate to obtai...
example 2
[0035] 1. Preparation of 4,4'-Didodecylphenyliodonium Hydrochloride
[0036] Take 100g of dodecylbenzene and 200mL of acetic anhydride into a 500mL three-necked reaction flask equipped with a thermometer and an electric stirrer, cool down to 10--5°C, then add 40g of potassium iodate, and add 60mL dropwise under stirring Concentrated sulfuric acid with a concentration of 98%, the rate of addition should keep the temperature not exceeding -5°C. After the dropwise addition, the reaction temperature rises to room temperature, and the reaction is continued for 5 hours; Separate the layers, extract the water layer twice with 100mL ether, combine the organic layers, add 50g barium chloride aqueous solution to the combined ether solution, a large amount of white precipitate precipitates, filter, wash with water, and dry the filtrate to obtain 4,4'-bis Dodecylphenyliodonium hydrochloride was then recrystallized from anhydrous isopropanol to obtain a white solid with a melting point of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com