Catalyst of hydrolytic perparing hydrogen by boron hydride and its preparation method
A borohydride and catalyst technology, applied in the field of catalysts, can solve the problems of uncontrollable hydrolysis reaction, increased catalyst cost, unsustainable reaction, etc., and achieves the effects of good catalytic effect, easy forming and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
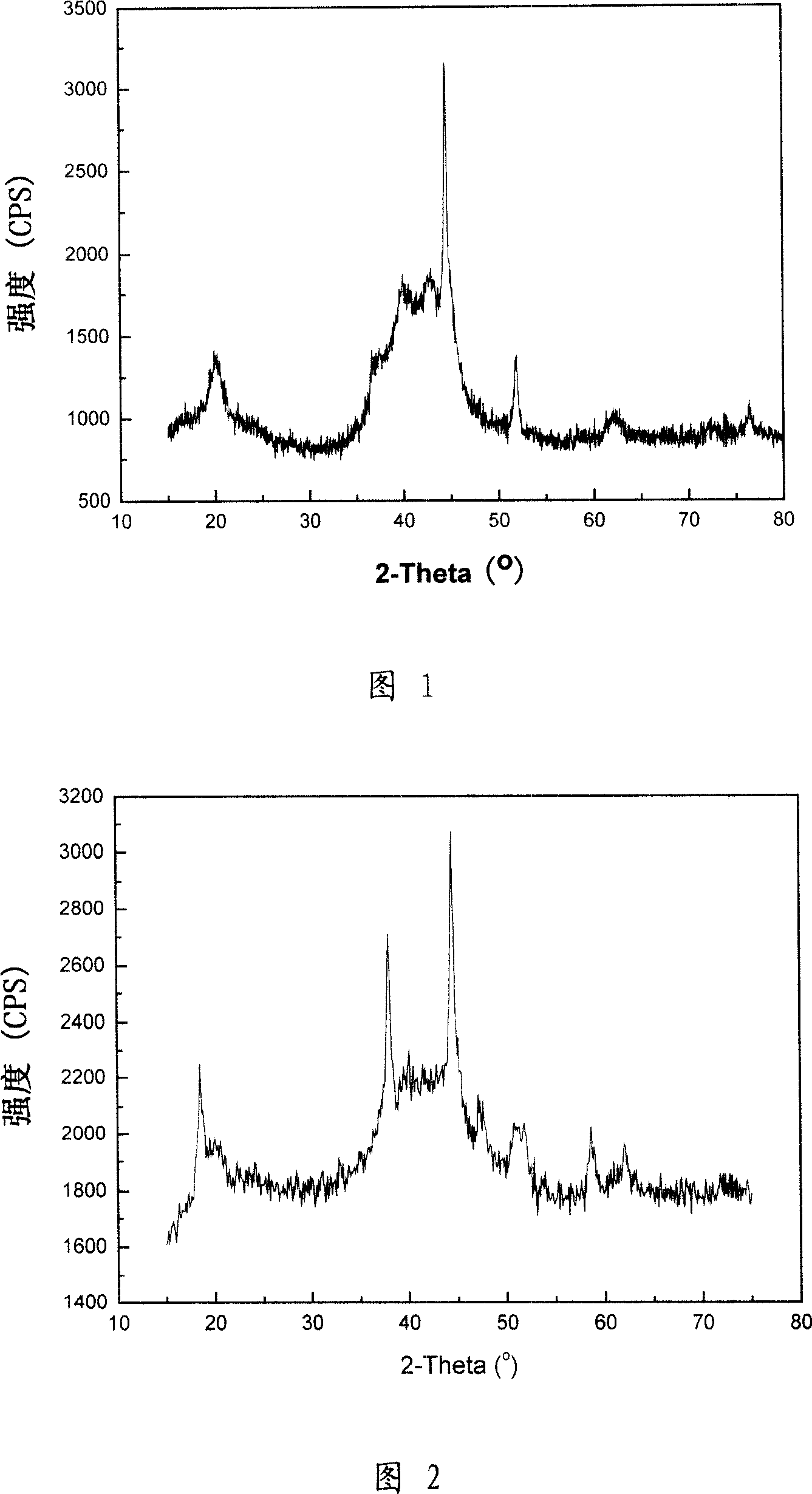
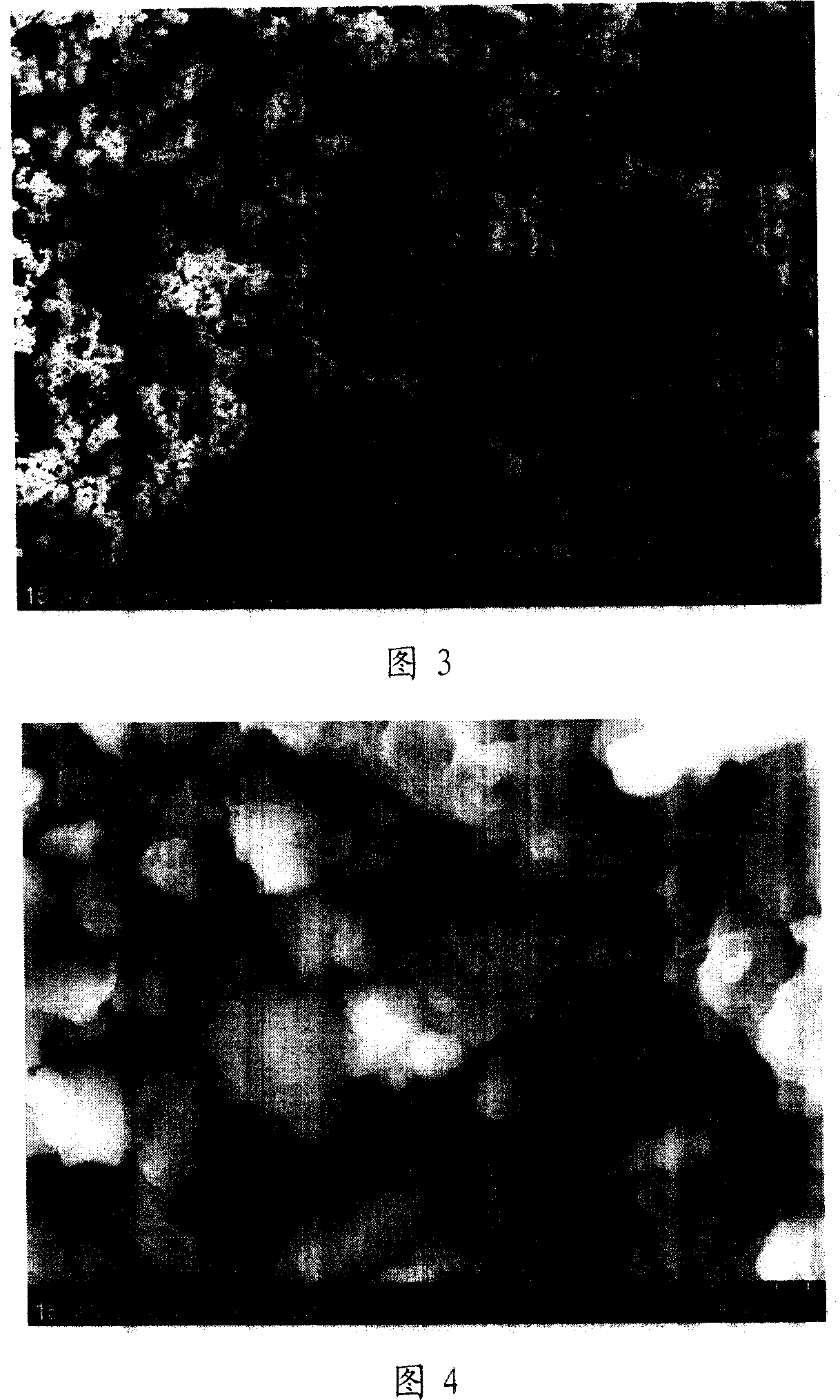
[0035] Take by weighing 4.30 grams of metal magnesium powder and 5.70 grams of metal nickel powder (atomic ratio Mg: Ni=2~2.05: 1), put into stainless steel ball mill jar, and add appropriate amount of steel balls (metal powder and steel ball mass ratio 1: 5 ~10), ball milled in an argon atmosphere for 60 hours. Every 12 hours, stop the ball mill, shovel the alloy from the wall of the ball mill, and continue ball milling after crushing. The obtained sample is detected through XRD (as shown in Figure 1), mainly by Mg 2 Ni alloy and a small amount of metallic nickel and magnesium oxide. The SEM images of the samples are shown in Figure 3 and Figure 4, and the size of the sample particles is approximately between 1 and 10 microns.
Embodiment 2
[0037] Take by weighing 0.5 gram of alloy sample made in 1 in the embodiment, immerse 10 milliliters of 1MCoCl 2 After 5 minutes in the solution, the reaction proceeds vigorously, and the Mg in the alloy will replace the cobalt ions in the solution, and metal cobalt is coated on the surface of the alloy particles. At the same time, the original magnesium oxide on the alloy surface will generate magnesium hydroxide. The XRD pattern and SEM pattern of the surface-treated sample are shown in Figure 2, Figure 5, and Figure 6, respectively.
[0038] Will go through CoCl 2 After pulverizing the surface-treated samples, add 10 ml of 1M NaBH 4 -1M NaOH aqueous solution, the hydrogen in the solution is released in the form of hydrogen gas very quickly, as shown in Figure 7.
Embodiment 3
[0040] Take by weighing the CoCl in embodiment 2 2 0.4 grams of surface-treated samples are pulverized, mixed with 0.1 grams of carbon black (acetylene black), 0.1 grams of 60% PTFE emulsion and a small amount of water, and mixed evenly. 2 After vacuum drying at 60°C on the foamed nickel substrate, it can be rolled and cut to make a sheet-shaped catalyst.
[0041] Put the catalyst into 10 ml of 1M NaBH 4 -In 1M NaOH solution, 500 ml of hydrogen can be released in 5 minutes, and the remaining about 350 ml of hydrogen can be released in about 5 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com