Nanometer particle target preparation of hydroxycamptothecine, and its preparing method
A hydroxycamptothecin and targeting technology, which is applied in the field of 10-hydroxycamptothecin nanoparticle targeting preparations and its preparation, can solve problems such as drug failure, and achieve improved curative effect, good targeting, and improved curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The formula that present embodiment adopts is as follows:
[0029] Gelatin 1.8g;
[0030] 10-Hydroxycamptothecin 1.7g;
[0031] 255ml of tert-butanol;
[0032] Vitamin C 40g;
[0033] Chitosan 0.30g;
[0034] Sodium carboxymethyl cellulose 6.50g;
[0035] Water for injection 765ml.
[0036] The preparation method is as described above.
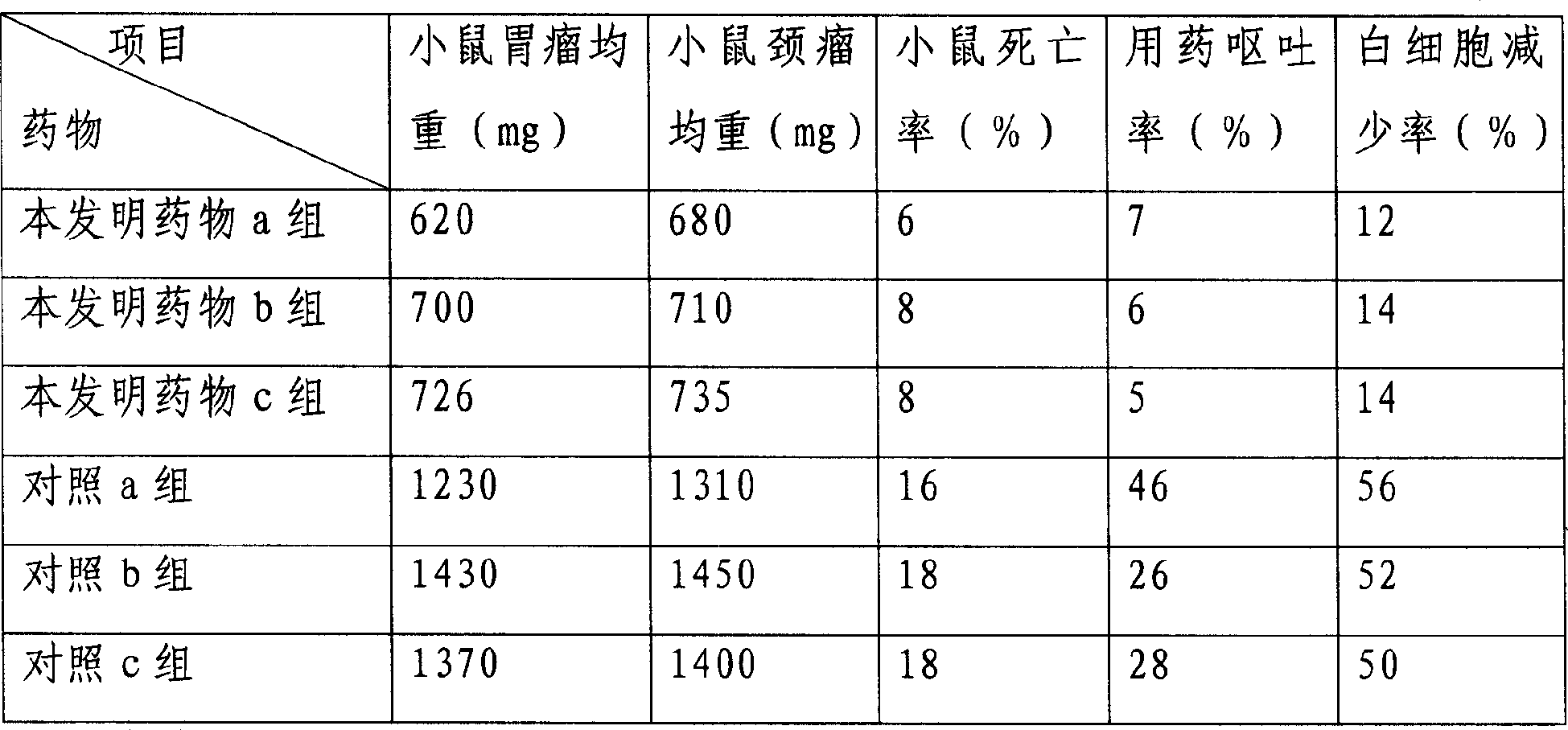
[0037] Pharmacodynamic experiment verification is as follows:
[0038] Mice models of gastric cancer and head and neck cancer were selected and randomly divided into 20 mice in each group.
[0039] Drug treatment group of the present invention is set as:
[0040] Drug group a of the present invention: 10-hydroxycamptothecin gelatin nanoparticles, the dosage is 1.5 mg / kg, twice a day, intraperitoneal injection, for 21 days.
[0041] Drug group b of the present invention: 10-hydroxycamptothecin gelatin nanoparticles, the dosage is 1.0 mg / kg, twice a day, intraperitoneal injection, medication for 21 days.
[0042] Drug group c of...
Embodiment 2
[0050] The formula that present embodiment adopts is as follows:
[0051] Human serum albumin 4.0g;
[0052] 10-Hydroxycamptothecin 3.2g;
[0053] 520ml tert-butanol;
[0054] Vitamin C 82g;
[0055] Chitosan 0.66g;
[0056] Sodium carboxymethyl cellulose 16g;
[0057] Water for injection 1560ml.
[0058] The preparation method is as described above.
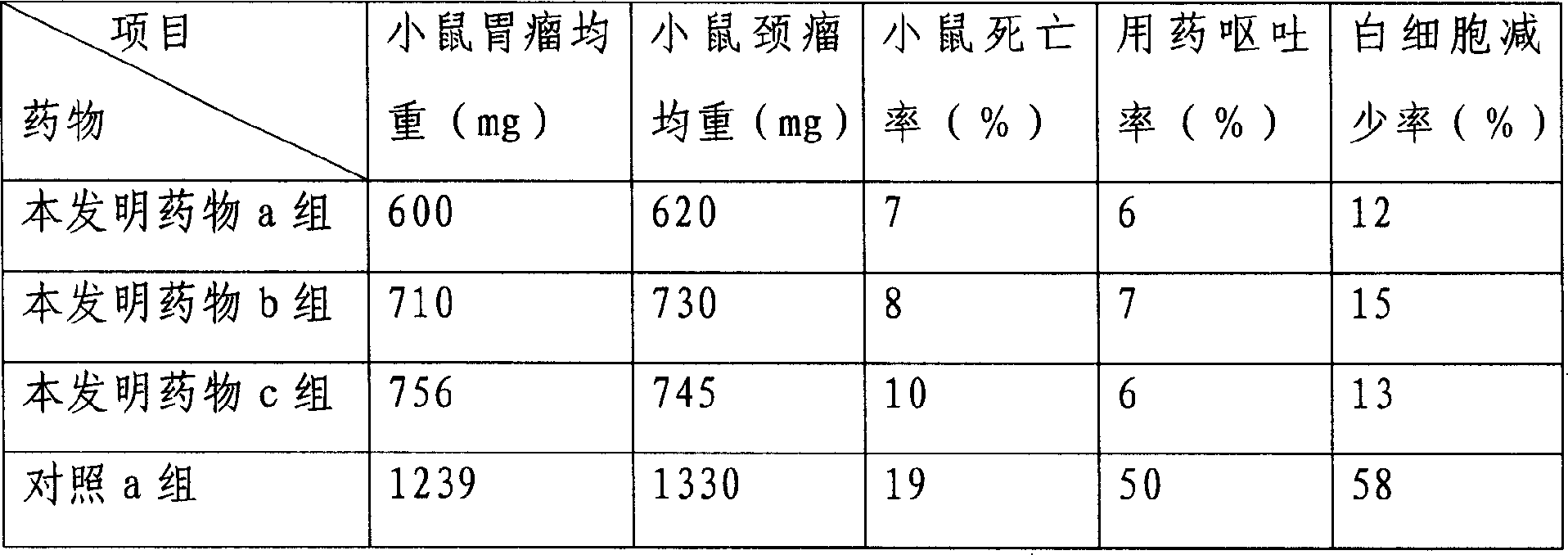
[0059] Pharmacodynamic experiment verification is as follows:
[0060] Mice models of gastric cancer and head and neck cancer were selected and randomly divided into 20 mice in each group.
[0061] Drug treatment group of the present invention is set as:
[0062] Drug group a of the present invention: 10-hydroxycamptothecin human serum albumin nanoparticles, the dosage is 1.5mg / kg, twice a day, intraperitoneal injection, medication for 21 days.
[0063] Drug group b of the present invention: 10-hydroxycamptothecin human serum albumin nanoparticles, the dosage is 1.0 mg / kg, twice a day, intraperitoneal injection, medicat...
Embodiment 3
[0072] The formula that present embodiment adopts is as follows:
[0073] Bovine serum albumin 5.4g;
[0074] 10-Hydroxycamptothecin 4.2g;
[0075] tert-butanol 630ml;
[0076] Vitamin C 120g;
[0077] Chitosan 0.90g;
[0078] Sodium carboxymethyl cellulose 20g;
[0079] Water for injection 1900ml.
[0080] The preparation method is as described above.
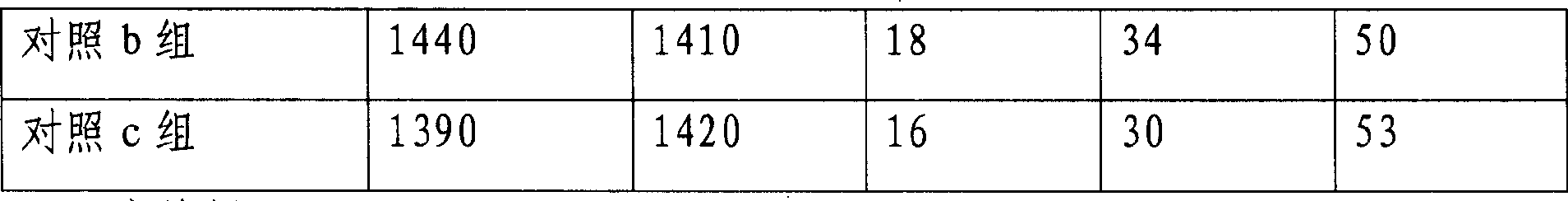
[0081] Pharmacodynamic experiment verification is as follows:
[0082] Mice models of gastric cancer and head and neck cancer were selected and randomly divided into 20 mice in each group.
[0083] Drug treatment group of the present invention is set as:
[0084] Drug group a of the present invention: 10-hydroxycamptothecin bovine serum albumin nanoparticles, the dosage is 1.5 mg / kg, twice a day, intraperitoneal injection, medication for 21 days.
[0085] Drug group b of the present invention: 10-hydroxycamptothecin bovine serum albumin nanoparticles, the dosage is 1.0 mg / kg, twice a day, intraperitoneal injection, medi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com