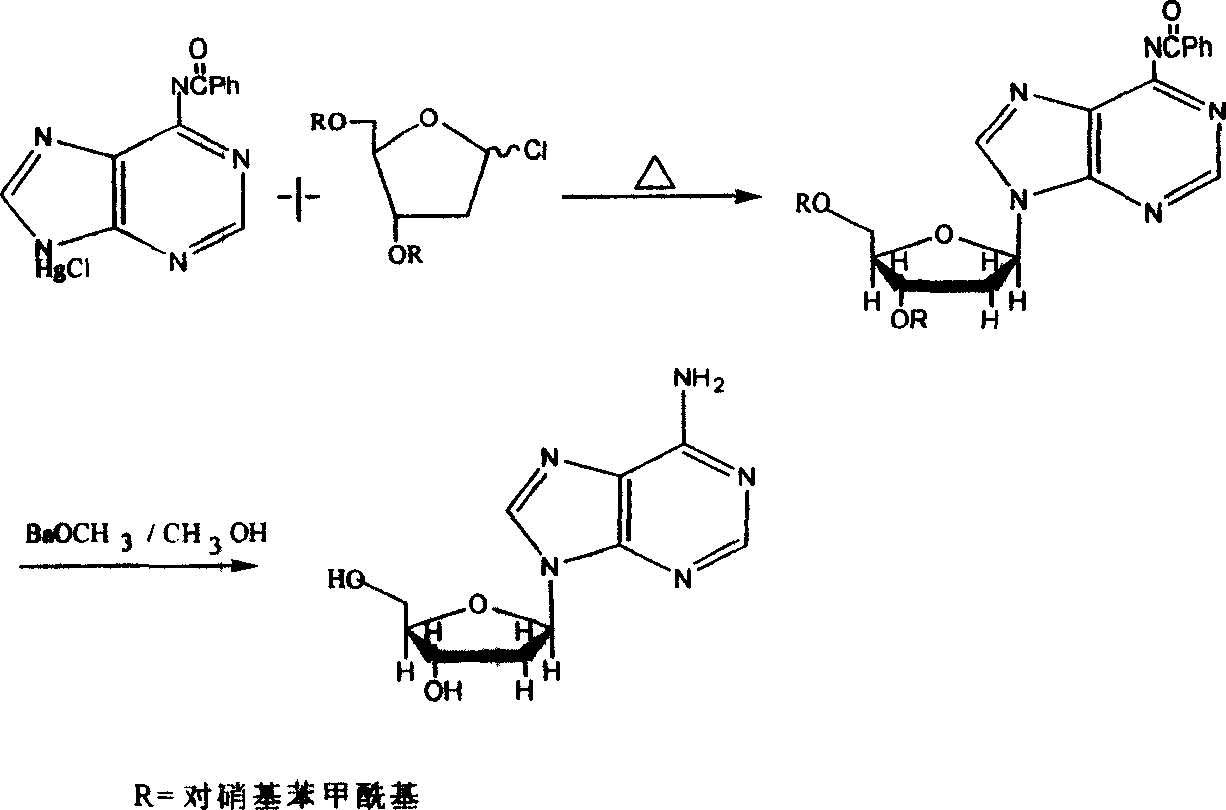
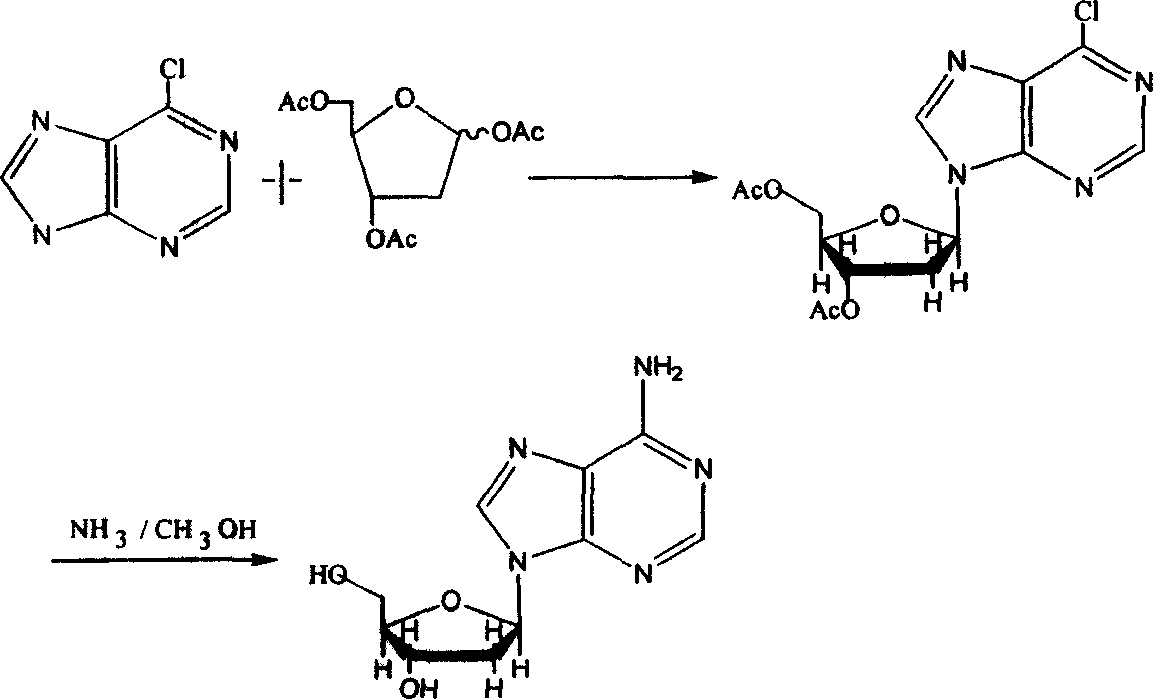
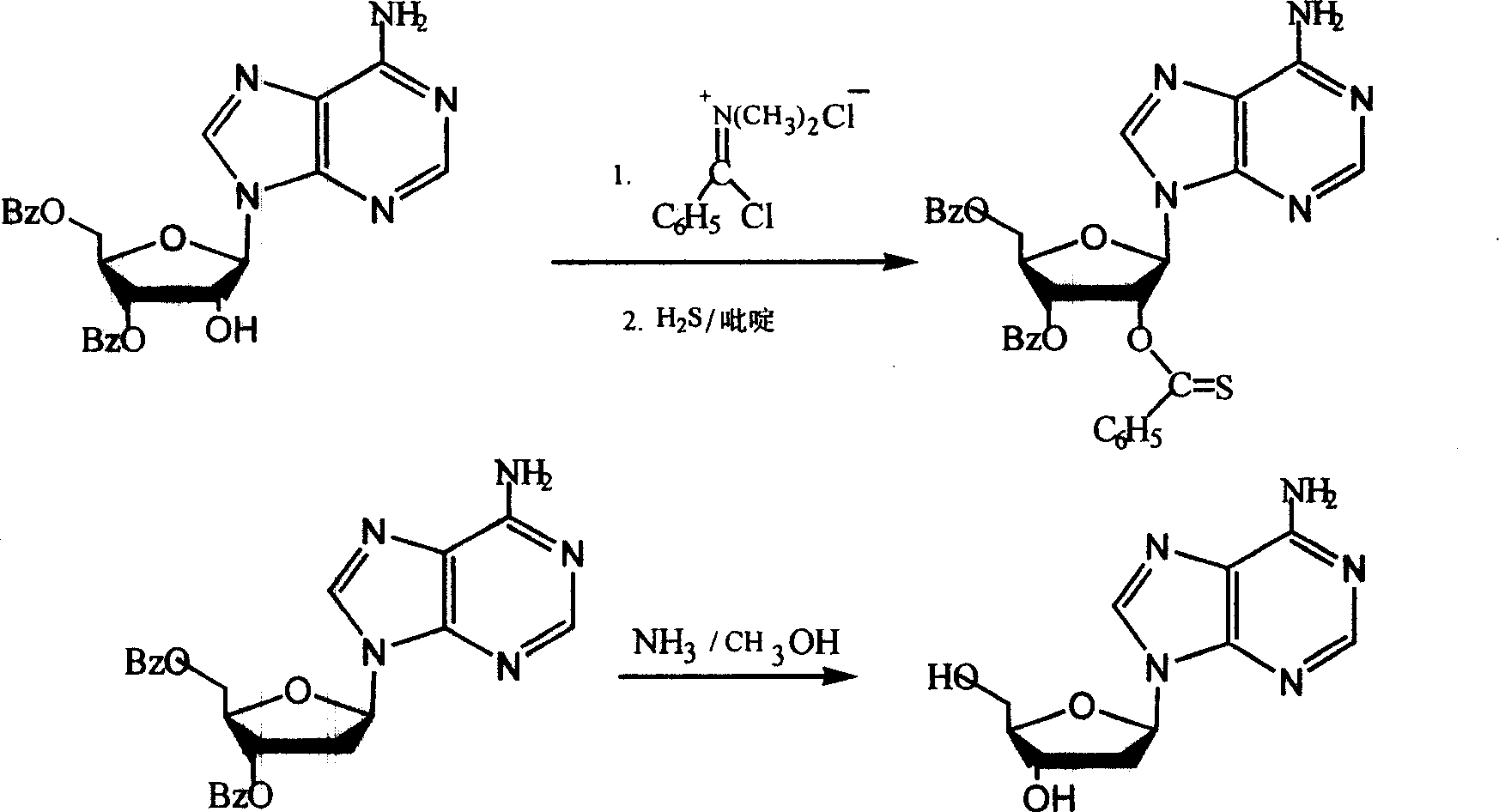
Chemical synthesis method for producing 2'-dexoyadenosine
A chemical synthesis method and technology of deoxyadenosine, which is applied in the technical field of chemical synthesis method for producing 2'-deoxyadenosine, can solve the problems of single configuration and short synthesis route, and achieve low price, stable product quality and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add 154kg 6-chloroadenine (I) and 1-acetyl-3,5-bis(p-methylbenzoyl)-2-deoxyribose (II) to a stainless steel reactor with a reflux device in 5000 liters ) 412kg, add 800 liters of dichloromethane containing 1-2kg two p-nitrophenol phosphate esters, heat to reflux and remove the acetic acid generated by the reaction in time, after the reaction is completed, connect the vacuum to remove the solvent, remove the vacuum, and obtain the intermediate (III ) 3'5'-bis(p-methylbenzoyl)-2'-deoxy-6-chloropurine nucleoside 380-430kg (conversion rate 75-85%).
[0036] (2) Dissolve 380 kg of intermediate (III) in 5 times the amount of ammonia-methanol solution, stir at room temperature for 3-6 hours, concentrate to dryness under reduced pressure, add 700 liters of distilled water to separate out the crude 2'-deoxyadenosine, and filter with suction. Then recrystallize with ethanol water to obtain 228-257 kg of 2'-deoxyadenosine product, the yield is 80-90%, and the total yield is 60...
Embodiment 2
[0038] (1) Add 154kg 6-chloroadenine (I) and 1-acetyl-3,5-bis(p-methylbenzoyl)-2-deoxyribose (II) to a stainless steel reactor with a reflux device in 5000 liters ) 412kg, add 800 liters of dichloromethane containing 1.5kg two p-fluorophenol phosphate esters, heat to reflux and remove the acetic acid generated by the reaction in time, after the reaction is completed, connect the vacuum to remove the solvent, remove the vacuum, and obtain the intermediate (III) 3',5'-bis(p-methylbenzoyl)-2'-deoxy-6-chloropurine nucleoside 309-355kg (conversion rate 61-70%).
[0039] (2) Dissolve 309 kg of intermediate (III) in 5 times the amount of ammonia-methanol solution, stir at room temperature for 3-6 hours, concentrate to dryness under reduced pressure, add 600 liters of distilled water to precipitate crude 2′-deoxyadenosine, and filter with suction. Then recrystallize with ethanol water to obtain 193-217 kg of 2'-deoxyadenosine product, the yield is 78-87%, and the total yield is 47.5-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com