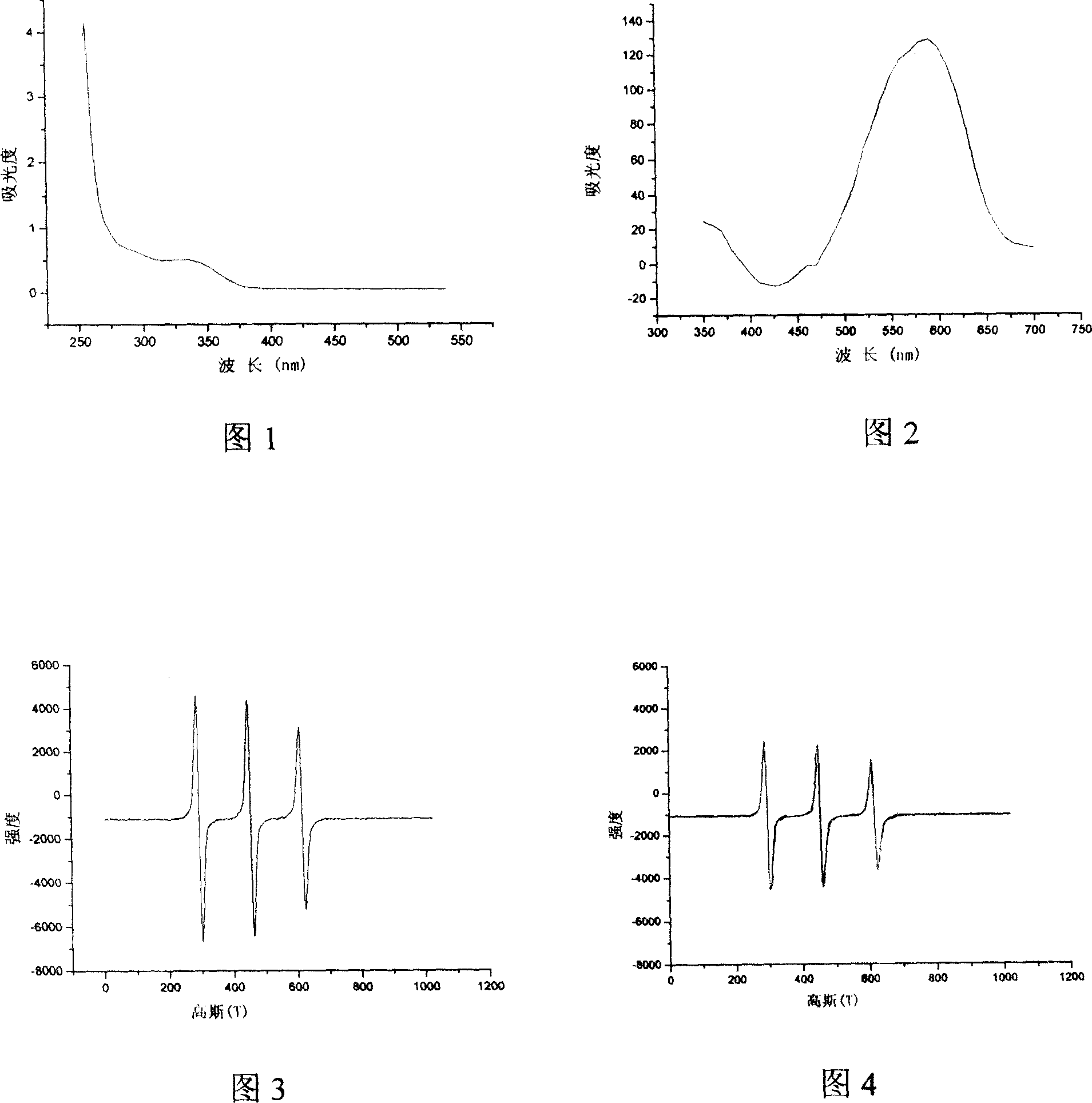
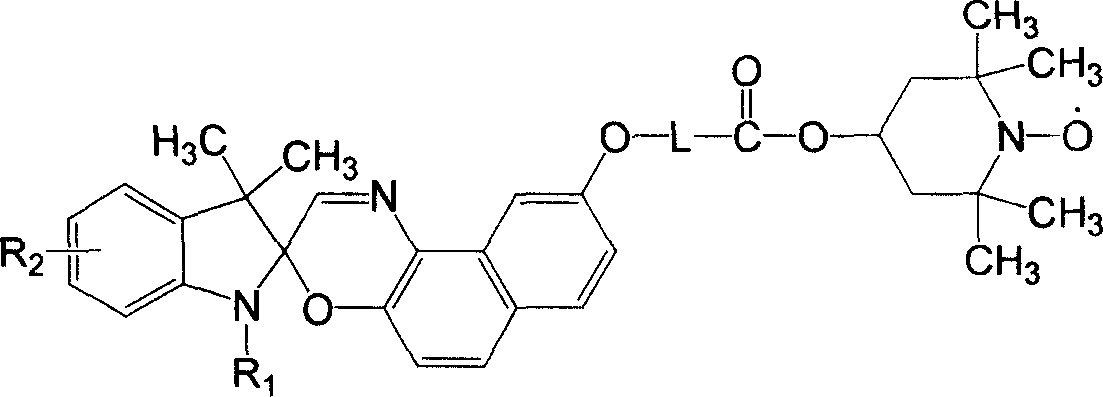
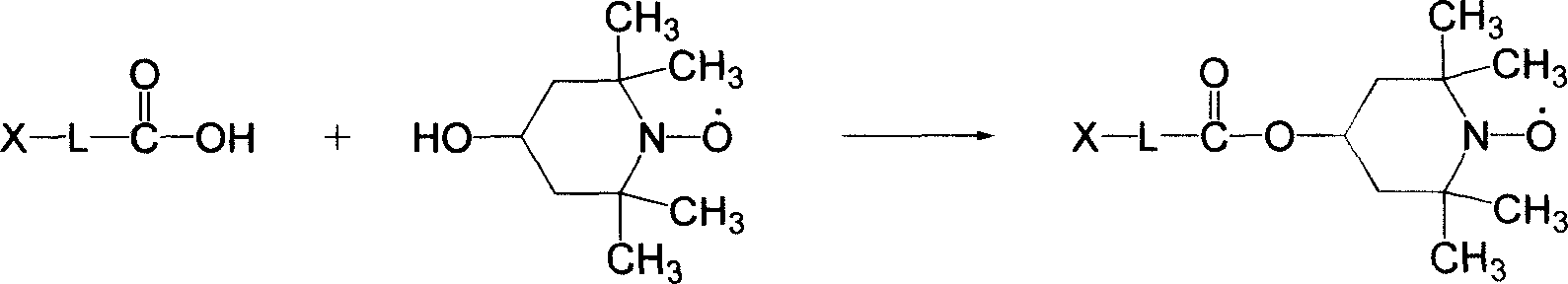Double functional photochromism compound with stable nitrogen-oxygen free radical group and spiro oxazinyl and synthetic method and use thereof
A photochromic and compound technology, used in chemical instruments and methods, organic chemistry, color-changing fluorescent materials, etc., can solve the problem of unreported organic photochromic molecules, and achieve simple and stable synthesis and purification methods. Good, easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 2,2,6,6-tetramethyl-1-oxopiperidin-4-2-chloroacetate:
[0042]
[0043] Add 10mmol of 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxygen, 10mmol of chloroacetic acid to 30ml of dichloromethane, and add an equimolar amount of Dicyclohexylcarbodiimide (DCC) and 0.6 mg of p-dimethylaminopyridine (DMAP) were reacted at room temperature for 12 hours. The formed urea was removed by filtration. The filtrate was successively washed with 10ml of 1M hydrochloric acid, 10ml of NaHCO 3saturated solution and 10ml of saturated brine for washing. Anhydrous Mg 2 SO 4 Dry overnight, filter, concentrate the filtrate, separate and purify with a silica gel column [v (ethyl acetate) / v (petroleum ether) = 3 / 5], and then recrystallize with petroleum ether to obtain red needle crystals with a yield of 89%.
Embodiment 2
[0045] Synthesis of 6-bromohexanoic acid 2,2,6,6-tetramethyl-1-oxopiperidin-4-ester:
[0046]
[0047] Add 10mmol of 4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxygen, 10mmol of 6-bromohexanoic acid to 30ml of dichloromethane, add at 0°C under nitrogen protection Equimolar amounts of dicyclohexylcarbodiimide (DCC) and 0.6 mg of p-dimethylaminopyridine (DMAP) were reacted at room temperature for 12 hours. The formed urea was removed by filtration. The filtrate was successively washed with 10ml of 1M hydrochloric acid, 10ml of NaHCO 3 saturated solution and 10ml of saturated brine for washing. Anhydrous Mg 2 SO 4 Dry overnight, filter, concentrate the filtrate, separate and purify with a silica gel column [v (ethyl acetate) / v (petroleum ether) = 3 / 5], and recrystallize with petroleum ether to obtain a red solid with a yield of 80%.
Embodiment 3
[0049]
[0050] 0.5mmol 1,3-dihydro-1,3,3-trimethyl-9'-hydroxy-spiro-[2H-indole-2,3'-[3H]naphtho[2,1-b][1 , 4] oxazine, 0.5mmol 2-chloroacetic acid 2,2,6,6-tetramethyl-1-oxopiperidine-4-ester (Example 1) and 6g anhydrous potassium carbonate, 25ml acetone, heated to reflux , reacted for 12 hours, filtered, the filtrate was concentrated, and silica gel column chromatography [v (ethyl acetate) / v (petroleum ether) = 2 / 5], the product was obtained with a yield of 30%.
[0051] 1 HNMR (CDCl 3 ): 7.87(1H, s), 7.72(2H, m), 7.63(1H, d, J=8.8Hz), 7.18(1H, d, J=8.8Hz), 7.12(1H, d, J=8.8Hz ), 6.94 (3H, m), 6.61 (1H, d, J=8, 8Hz), 4.88 (2H, s), 2.79 (3H, s), 1.39 (6H, s).
[0052] MS: m / z 556.8 [M + ].
[0053] Paramagnetic (ESR) (CH 2 Cl 2 , X-band, room temperature): triplet (1:1:1), g=2.007, a=15.34G.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com