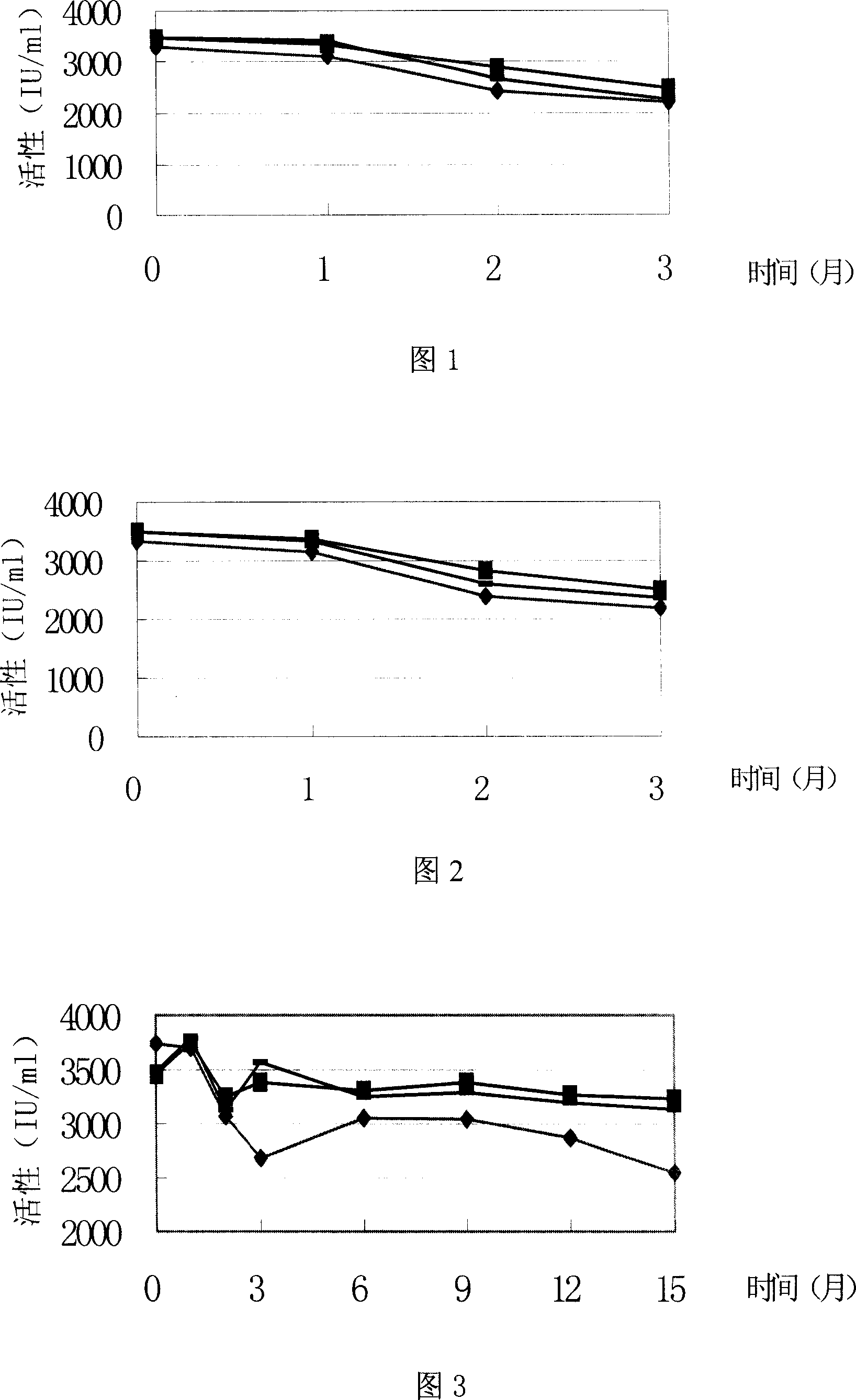
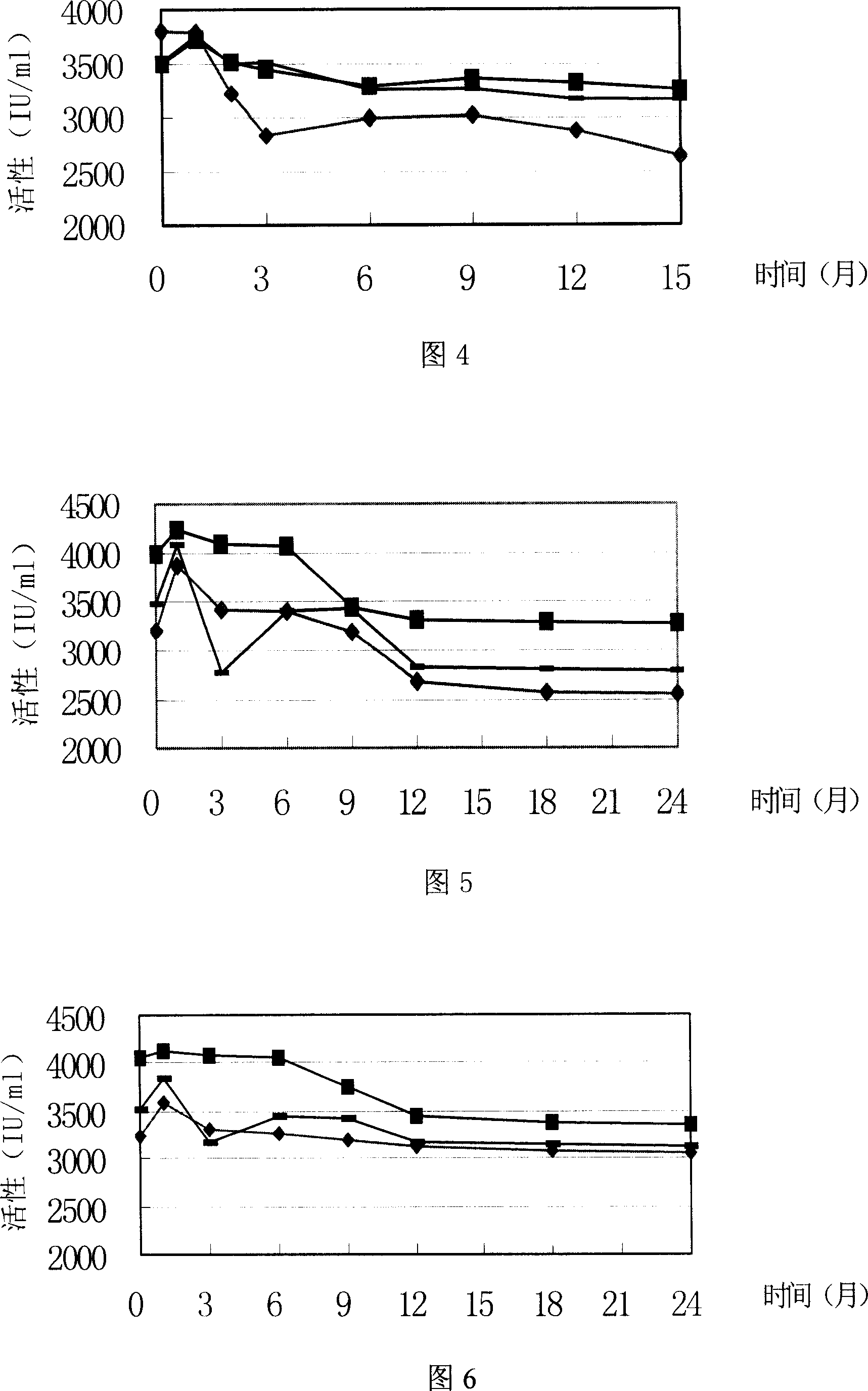
Recombinant erythropoietin liquor preparation
A technology of recombinant human erythropoietin and solution, which can be used in extracellular fluid diseases, medical preparations containing active ingredients, blood diseases, etc. Purity and stability, high activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of 3000IU / ml rhEPO containing rHSA
[0021] formula:
[0022] Component Concentration Dosage
[0023] rhEP0 3000IU / ml 165ml
[0024] rHSA 2.5mg / ml 137.5ml
[0025] Sodium citrate, chloride Sodium citrate 20mmol / L, sodium chloride
[0026] 10697.5ml
[0027] Sodium buffer 100mmol / L (adjust pH to 6.9)
[0028]
[0029] 10000 sticks
[0030] Take rhEPO stock solution (2.0×10 5 IU / ml) 165ml, add rHSA (200mg / ml) 137.5ml, add sodium citrate, sodium chloride buffer 10697.5ml. After fully mixing, aseptically filter into a sterilized container, which is the semi-finished product of rhEPO.
[0031] Then fill the semi-finished solution into 3ml vials, and add 1.1ml to each bottle. Finally, stoppering, capping, and labeling were performed to make 3000 IU / branch of rhEPO injection.
Embodiment 2
[0032] Embodiment 2: the preparation of 1500IU / ml rhEPO containing rHSA
[0033] formula:
[0034] Ingredients Content Dosage
[0035] rhEPO 1500IU / ml 82.5ml
[0036] rHSA 2.5mg / ml 137.5ml
[0037] Sodium citrate, chloride Sodium citrate 20mmol / L, sodium chloride
[0038] 10780ml
[0039] Sodium buffer 100mmol / L (adjust pH to 6.9)
[0040]
[0041] 10000 sticks
[0042] Preparation method is the same as embodiment 1
Embodiment 3
[0043] Embodiment 3: the preparation of 6000IU / ml rhEPO containing rHSA
[0044] formula:
[0045] Ingredients Content Dosage
[0046] rhEPO 6000IU / ml 330ml
[0047] rHSA 4.0mg / ml 220ml
[0048] Sodium citrate, chloride Sodium citrate 20mmol / L, sodium chloride
[0049] 10450ml
[0050] Sodium buffer 100mmol / L (adjust pH to 6.9)
[0051]
[0052] 10000 sticks
[0053] Preparation method is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com