Triazine series oligomer and its synthesizing method
A synthesis method and oligomer technology, applied in the direction of organic chemistry, etc., can solve the problems of poor thermal stability of products, large amount of organic solvent, discontinuous reaction process, etc., and achieve good thermal stability, less amount of organic solvent, solvent The effect of convenient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
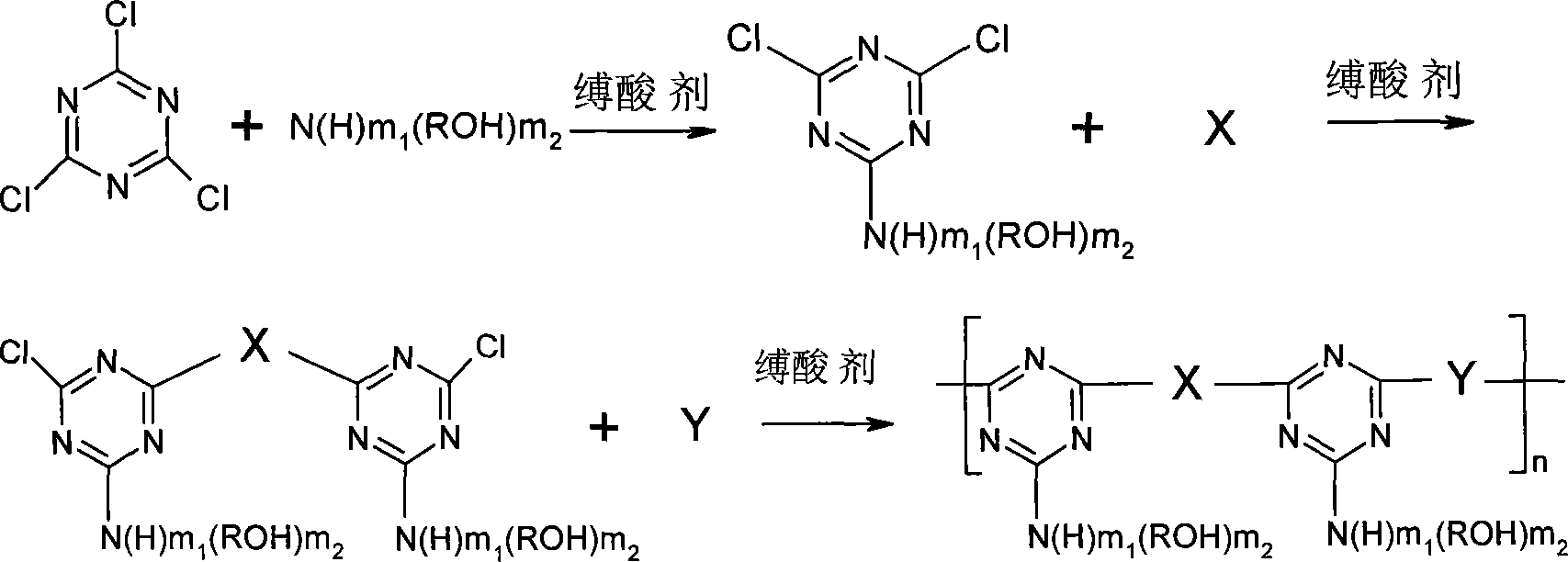
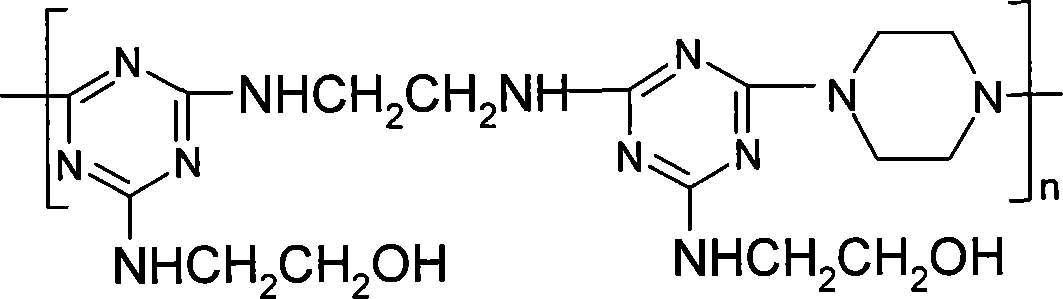
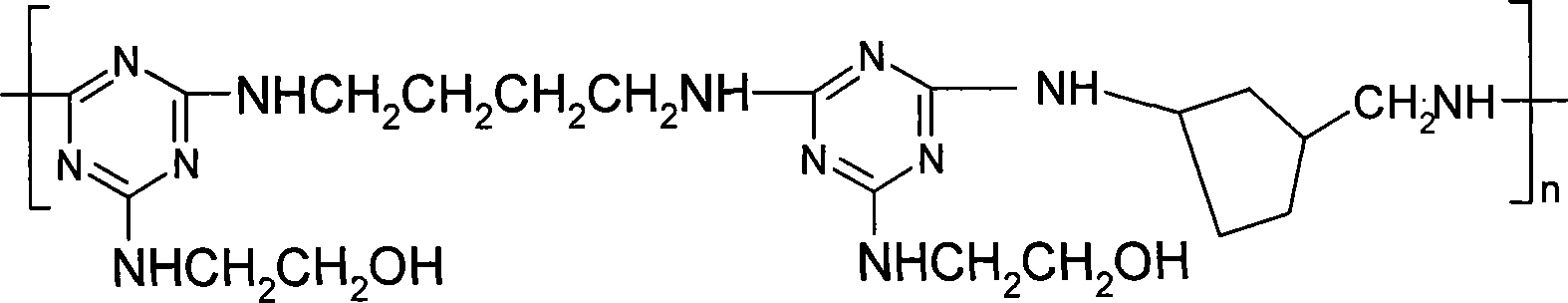
[0015] Specific embodiment 1: The general structure of the triazine-based oligomer of this embodiment is:
[0016] Where: m 1 =0 or 1, m 2 = 1 or 2; R = (CH 2 ) n1 , N1=1~6; X means NHR 1 NH or Or Where R 1 =(CH 2 ) n2 Or benzene ring, n2=2~6; Y represents piperazine or Or m=1-15.
specific Embodiment approach 2
[0017] Embodiment 2: The steps of the method for synthesizing triazine-based oligomers in this embodiment are as follows: a. Using cyanuric chloride as a starting material and inorganic base as an acid binding agent, adding cyanuric chloride into a reaction vessel, Add solvent to the reaction vessel to make cyanuric chloride evenly dispersed. Add alcoholamine and acid binding agent dropwise to the reaction vessel under the condition of 0~10℃ to control the dropping rate of acid binding agent to control the pH value of the solution. After 5~7, after 2~4 hours of reaction, when the pH value of the reaction solution is close to neutral, the first step substitution reaction is over, and the monosubstituted cyanuric chloride 2-hydroxyalkylamino-4,6-dichloride is produced. -1,3,5-triazine, in which the ratio of cyanuric chloride and alcohol amine is 1:1; b. Raise the temperature to 40-70°C, and add dropwise the two used in the second step of the substitution reaction. The pH value of am...
specific Embodiment approach 3
[0019] Embodiment 3: In this embodiment, in step one, the solvent is acetone, water or a mixture of acetone and water. Others are the same as the second embodiment.
[0020] In this embodiment, when the solvent is a mixture, acetone and water are mixed in an arbitrary ratio.
PUM
| Property | Measurement | Unit |
|---|---|---|
| oxygen index | aaaaa | aaaaa |
| oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com