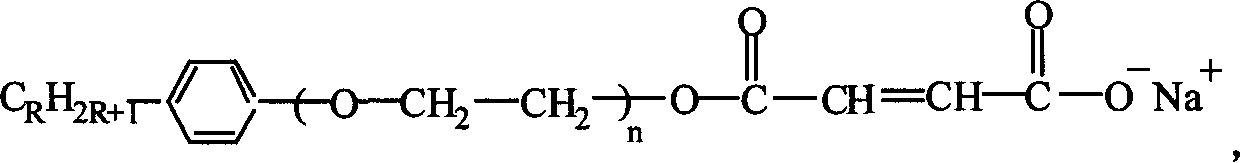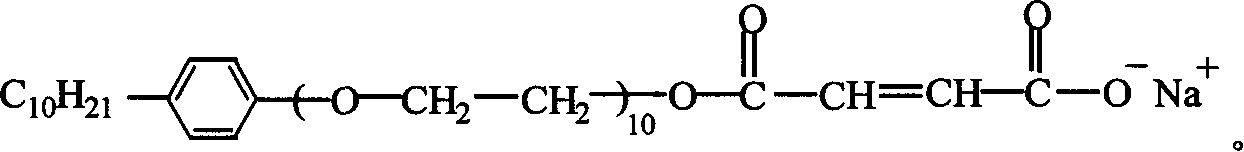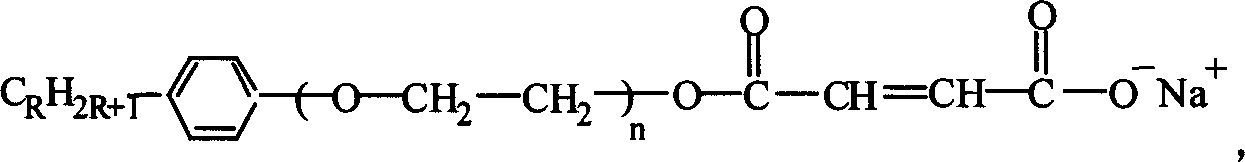Fluoride core-shell acrylic-resin emulsion, its production and use
A core-shell acrylate and acrylate technology, applied in the field of polymer chemistry, can solve the problems of coating water resistance, stain resistance limitation, harsh reaction conditions, high film forming temperature, etc., to achieve high hydrophobicity, low film forming temperature, good flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of reactive emulsifier
[0033] Take 4.75Kg of maleic anhydride, 3Kg of OP-10, a kind of alkylphenol-based polyoxyethylene ether, mix and react at 70~130℃ for 4~6 hours, then add sodium carbonate to adjust the PH value to 6. ~7, to prepare a reactive emulsifier. The molecular formula of the reactive emulsifier is:
[0034]
[0035] Using other alkylphenol-based polyoxyethylene ethers, such as OP-2~OP-15, OP-18, OP-20, etc., instead of OP-10, the reactive emulsifier can also be prepared. The molecular structure of the reactive emulsifier and the effect of preparing fluorine-containing core-shell acrylate emulsion are roughly the same as using OP-10.
[0036] In the preparation, the alkylphenol-based polyoxyethylene ether with better effect is nonylphenol-based polyoxyethylene ether (OP-10).
Embodiment 2
[0037] Example 2 Preparation of fluorine-containing core-shell acrylate emulsion
[0038] The preparation method of fluorine-containing core-shell acrylate emulsion includes two steps: reactive emulsifier preparation and fluorine-containing emulsion preparation.
[0039] See Example 1 for the preparation of reactive emulsifiers.
[0040] Preparation of fluorine-containing emulsion: take 0.5Kg of reactive emulsifier, 13Kg of methyl methacrylate and 8Kg of butyl acrylate to form a nucleus. Test the conversion rate during the reaction. When the nucleus monomer is about 85% polymerized, add methyl 8Kg of methyl acrylate, 13Kg of butyl acrylate, and 6Kg of dodecafluoroheptyl methacrylate. The reaction was continued for 2 hours to form a shell outside the core.
Embodiment 3
[0041] Example 3 Preparation of fluorine-containing core-shell acrylate emulsion
[0042] See Example 1 for the preparation of reactive emulsifiers.
[0043] Preparation of fluorine-containing emulsion: take 0.5Kg of reactive emulsifier, add 6.5Kg of methyl methacrylate, 4Kg of butyl acrylate, 5.5Kg of ethyl methacrylate, and 6Kg of butyl methacrylate to copolymerize four acrylate monomers. The reaction forms a nucleus. The conversion rate was tested in the reaction. When the core monomer was polymerized to be equal to or greater than 80%, 4Kg of methyl methacrylate, 6Kg of butyl acrylate, 4Kg of ethyl methacrylate, 3Kg of butyl methacrylate and methyl were added. Dodecafluoroheptyl acrylate 3Kg, five monomers are copolymerized outside the core to form a shell, and the reaction time is about 4 hours.
[0044] The effect of preparing the fluorine-containing core-shell acrylate emulsion is basically the same as that of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com