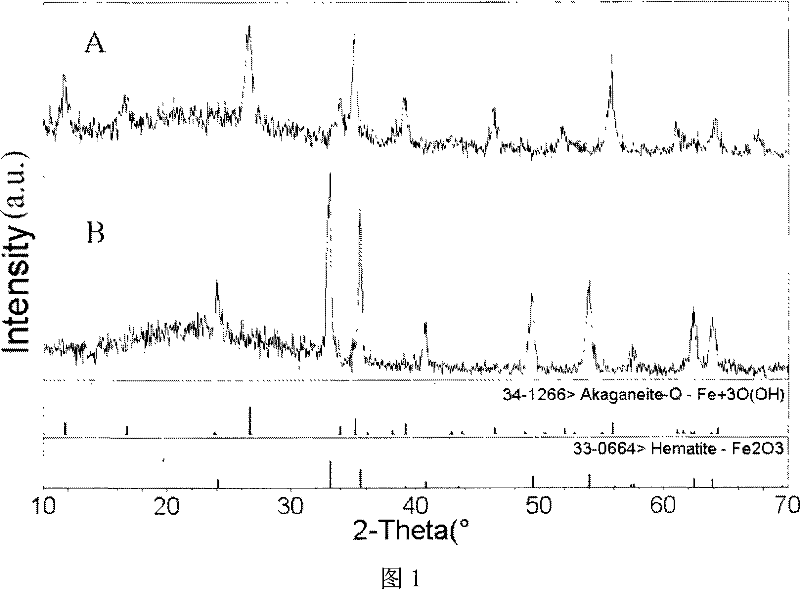
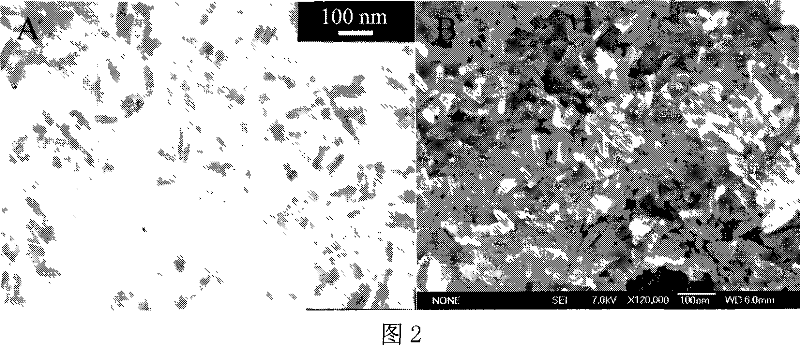
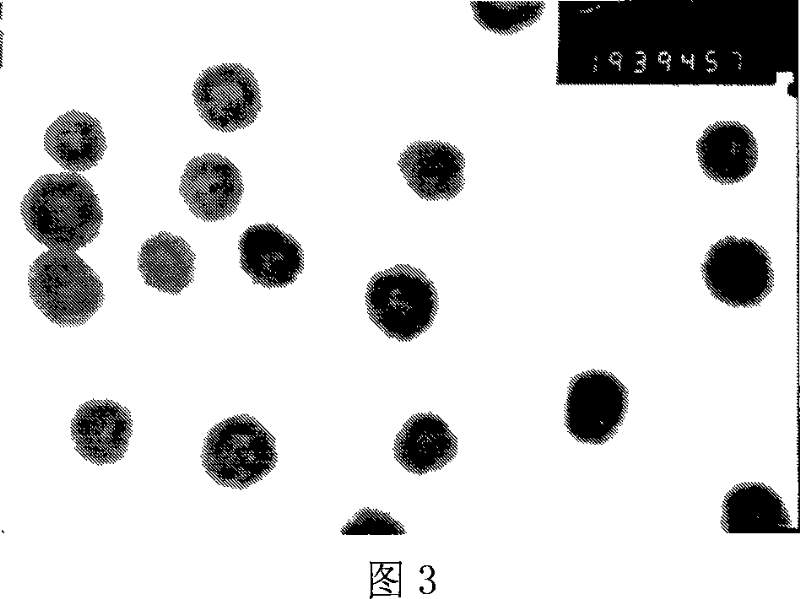
Method for preparing radius-controllable ferric oxide hollow ball
A technology of iron oxide and iron oxide nanometers, which is applied in the field of chemical materials, can solve the problems of inability to quickly adjust the particle size of the product, unfavorable industrial production, and difficulty in product separation, and achieve products with strong controllability, excellent product crystallization, and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: First, the aqueous solution containing 0.1M ferric chloride and 0.15M urea is mixed with ethanol (alcohol / water volume ratio=5:1) and then packed into a three-necked flask; heated and refluxed for 8 hours under continuous stirring; the obtained The product was separated by suction filtration, washed three times with ethanol and distilled water, and then dried in an oven at 60°C to obtain iron hydroxide nanorods; the iron hydroxide nanorods accounting for 0.05% by weight of the ethanol / water mixed system were dispersed Stir evenly in an ethanol / water mixed system with a volume ratio of 20:1, then put it into a stainless steel reactor with a filling degree of 80%; treat it at 160°C for 8 hours, cool it naturally, wash the product with ethanol and distilled water 3 times, and put it in an oven Iron oxide hollow spheres can be obtained after medium drying.
Embodiment 2
[0029] Example 2: First, mix the aqueous solution containing 0.3M ferric chloride and 0.45M urea with n-propanol (alcohol / water volume ratio = 1: 1) and put it into a three-necked flask; heat and reflux for 4 hours under continuous stirring; The obtained product was separated by suction filtration, and the product was washed with ethanol and distilled water for 3 times, and then dried in an oven at 60°C to obtain iron hydroxide nanorods; Disperse in an ethanol / water mixed system with a volume ratio of 30:1 and stir evenly, then put it into a stainless steel reactor with a filling degree of 70%; treat it at 180°C for 10 hours, cool naturally, wash the product three times with ethanol and distilled water, and then put it in After drying in an oven, iron oxide hollow spheres can be obtained.
Embodiment 3
[0030] Embodiment 3: first will prepare the aqueous solution containing 0.3M ferric chloride and 0.45M urea and isopropanol (alcohol / water volume ratio=1: 5) and mix and then pack into a three-necked flask; heat and reflux for 4 hours under continuous stirring The obtained product is separated by suction filtration, and the product is washed 3 times with ethanol and distilled water, and then dried in an oven at 60°C to obtain iron hydroxide nanorods; the iron hydroxide nanorods accounting for 4.0% of the ethanol / water mixed system Disperse the rods in an ethanol / water mixed system with a volume ratio of 10:1 and stir evenly, then put them into a stainless steel reactor with a filling degree of 80%; treat at 200°C for 4 hours, cool naturally, and wash the product 3 times with ethanol and distilled water Iron oxide hollow spheres can be obtained after drying in an oven.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com