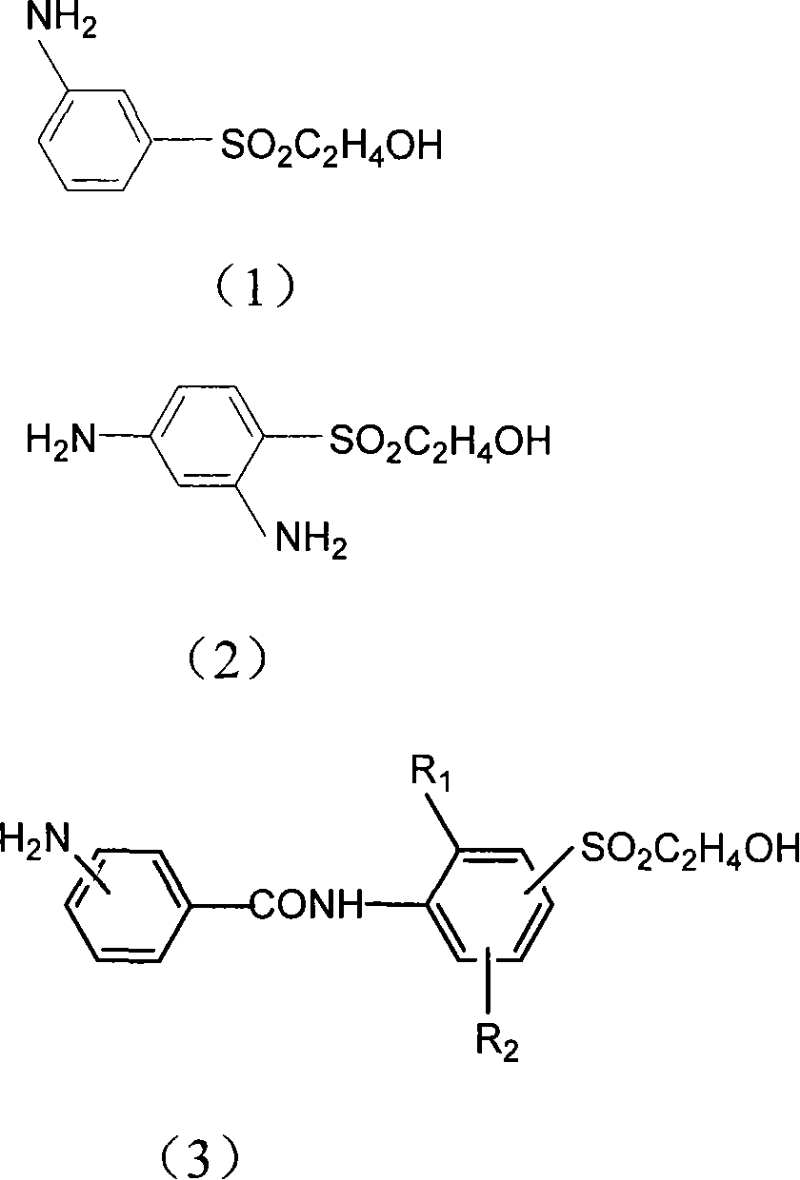
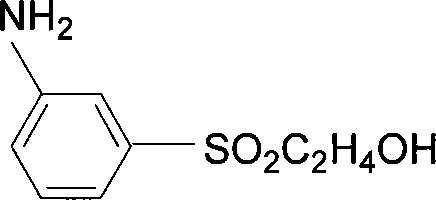
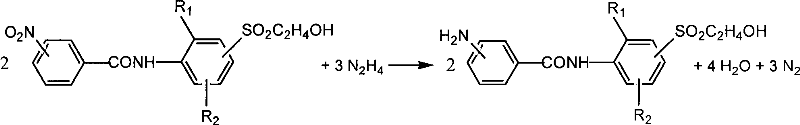Preparation method of aniline derivative containing 2-hydroxyethylsulfonyl
A technology of hydroxyethylsulfone-based aniline and derivatives, which is applied in the field of fine chemical industry, can solve the problems of large discharge of waste water and waste residue, low product quality, and pollution of three wastes, etc., and achieves simple equipment, high product yield and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: 69.3g (0.3mol) 3-(2-hydroxyethyl sulfone group) nitrobenzene is joined in 700ml methyl alcohol, is warming up to 65 ℃, adds 1.4g 0.5% palladium carbon, drips 28ml hydrazine hydrate ( Content 80%). Reflux at this temperature for 6-8 hours, use thin layer chromatography in developing solvent: chloroform: acetone = 8: 2 (v / v) to detect the disappearance of raw materials, which is the end point of reduction. The palladium carbon was removed by filtration, the filtrate was evaporated to dryness under reduced pressure (the distilled methanol was recovered and used mechanically), the residue was diluted with water, filtered to obtain a filter cake, and dried to obtain 59 g of the product, with a yield of 98%.
Embodiment 2
[0058] Embodiment 2: 86g (0.2mol) 4-nitro-N-[2-sulfonic acid group-4-(2-hydroxyethyl sulfone group) phenyl) benzamide is added to 650mol water and 50ml dimethyl In formamide, heat up to 100°C, add 5g of ferric trichloride-carbon, dropwise add 20ml of hydrazine hydrate, and reflux at 98-100°C for 10-12 hours. After the reaction reached the end point, the catalyst was removed by filtration, and the solvent was evaporated to obtain 72 g of solid, which was the product, with a yield of 90%.
Embodiment 3
[0059] Embodiment 3: 87.5g 4-nitro-N-[3-(2-hydroxyethyl sulfone group) phenyl) benzamide is added in 700ml ethanol, is warmed up to 78 ℃, adds 45ml hydrazine hydrate (content 50 %) and 3.5g ferric oxyhydroxide, reflux at 78-80°C for 8-10 hours. After the reaction reached the end point, the catalyst was removed by filtration while it was hot. After the filtrate was cooled, light off-white crystals were precipitated. After drying, 72 g of dry product was obtained, with a yield of 90%. The collected filtrate can be applied directly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com