Method of preparing nano-crystal titanium oxide colloid used for optoelectronic cell
A technology of nanocrystals and photovoltaic cells, applied in the field of nanomaterials, can solve the problems of incomplete crystallization, instability, and easy stratification of nano-TiO sol, and achieve the effects of rapid response, improved conversion efficiency, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
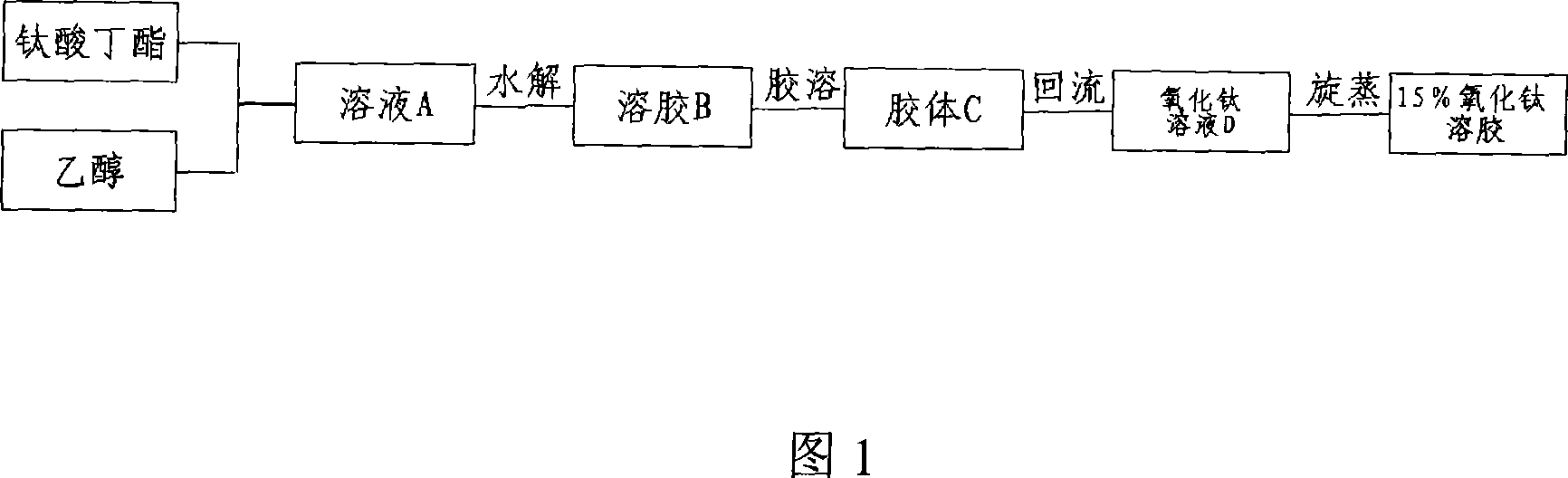
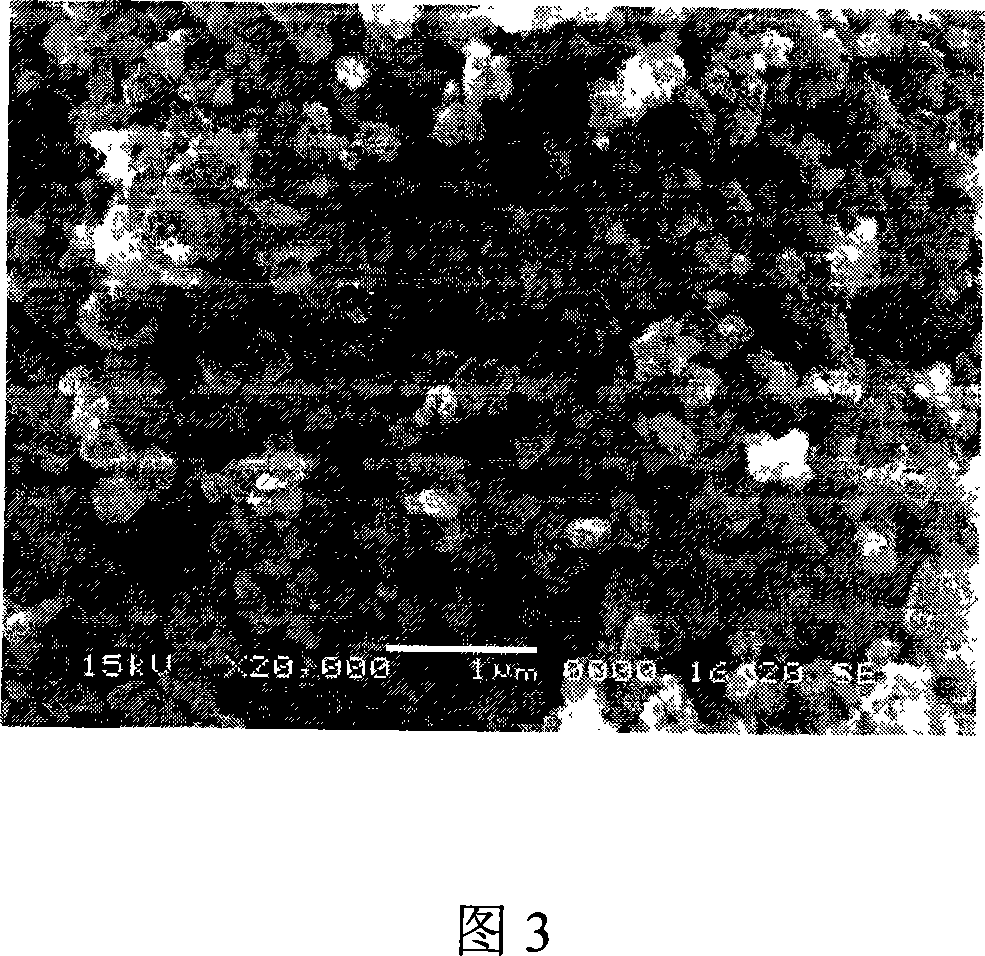
[0031] First prepare solution A: add 100 mL of butyl titanate to 200 mL of ethanol solution, then add 20 mL of diethanolamine as a stabilizer, stir vigorously to obtain a light yellow transparent solution. Slowly add 25mL of pure water dropwise into solution A while vigorously stirring to form transparent sol B. Put sol B at a constant temperature of 25°C for 24 hours to obtain uniform TiO 2 Colloid C. TiO 2 Colloid C was refluxed at 150 ° C for 5 h to evaporate to remove residual organic matter, and to obtain nanocrystalline TiO with a mass concentration of about 1%. 2 Solution D. Finally, the solution D was rotary vacuum evaporated for 2h to obtain nanocrystalline TiO with a mass concentration of 15%. 2 colloid. Fig. 2 is the TiO prepared in this embodiment 2 Spherical TiO can be seen in the TEM photo of the colloid after drying 2 Particles, the particle size of which is less than 10nm. Figure 3 is TiO 2 SEM photo of the colloidal coating, nano-TiO can be found 2Th...
Embodiment 2
[0033] First prepare solution A: add 150 mL of butyl titanate to 300 mL of ethanol solution, then add 30 mL of diethanolamine as a stabilizer, stir vigorously to obtain a light yellow transparent solution. Slowly add 37.5mL of pure water dropwise into solution A while vigorously stirring to form transparent sol B. Put sol B at a constant temperature of 25°C for 24 hours to obtain uniform TiO 2 Colloid C. TiO 2 Colloid C was refluxed at 100°C for 2h and evaporated to remove residual organic matter to obtain TiO with a mass concentration of about 1%. 2 Solution D. Add dilute hydrochloric acid to adjust TiO 2 The pH value of the solution was 9-11, and then the solution D was evaporated in a rotary vacuum for 2 hours to obtain nanocrystalline TiO with a mass concentration of 15%. 2 colloid. Description of the prepared nanocrystalline TiO 2 The colloid can adjust different pH values, and it can still maintain uniformity and stability in a weakly acidic environment.
Embodiment 3
[0035] First prepare solution A: add 100 mL of butyl titanate to 200 mL of ethanol solution, then add 20 mL of acrylamide as a stabilizer, stir vigorously to obtain a light yellow transparent solution. Slowly add 25mL of pure water dropwise into solution A while vigorously stirring to form transparent sol B. Put sol B at a constant temperature of 25°C for 24 hours to obtain uniform TiO 2 Colloid C. TiO 2 Colloid C was refluxed at 100°C for 2h and evaporated to remove residual organic matter to obtain TiO with a mass concentration of about 1%. 2 solution. Fig. 6 is the TiO prepared in this embodiment 2 From the XRD spectrum of the sample, it can be seen that the crystallization of titanium oxide is not complete, and there are only a few anatase phase components, indicating that strict control of the reflow temperature and time of the colloid is very important for the crystallization of the sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com