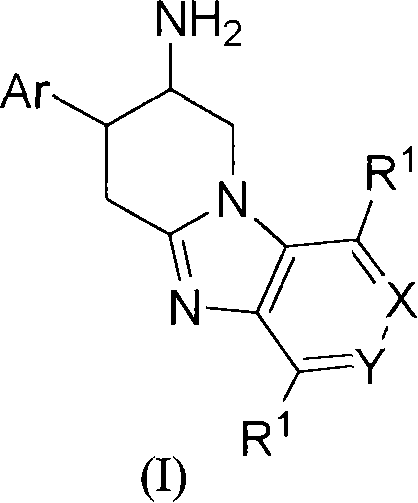
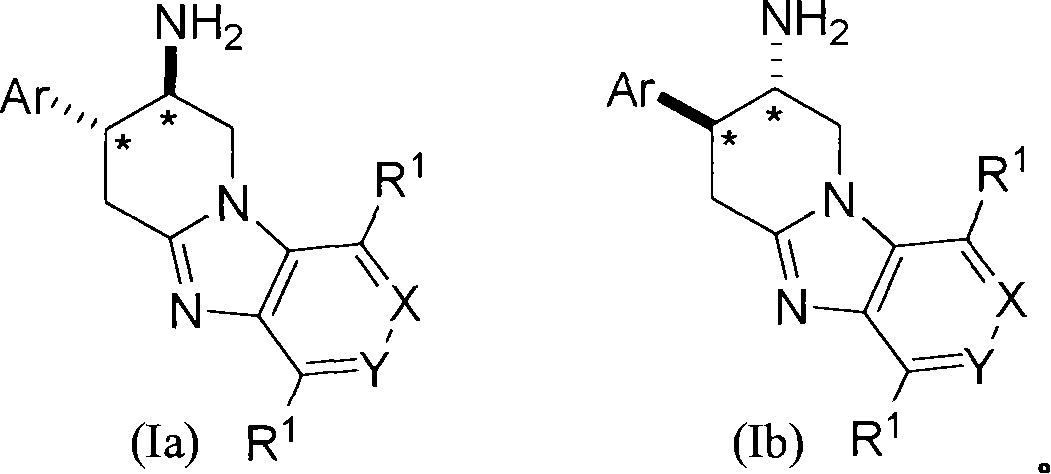
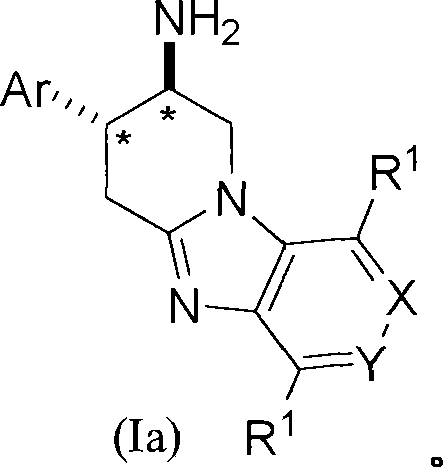
Fused aminopiperidines as dipeptidyl peptidase-iv inhibitors for the treatment or prevention of diabetes
An alkyl, hydroxyl technology, applied in the field of substituted fused amino piperidines, can solve problems such as DPP-IV inhibitors that have not been extensively studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240]
[0241] (2R,3R)-7-(pyrrolidin-1-ylcarbonyl)-3-(2,4,5-trifluorophenyl)-1,2,3,4-tetrahydropyrido-[1,2 -a] Benzimidazol-2-amine bis(trifluoroacetic acid) salt
[0242] Step A: 4-[5-[(tert-Butoxycarbonyl)amino]-2-oxo-4-(2,4,5-trifluorophenyl)piperidin-1-yl]-3-nitrobenzene Methyl formate
[0243] A septum-capped dry flask was charged with 1.0 g (2.9 mmol) of intermediate 1, 1.1 g (4.4 mmol) of methyl 4-bromo-3-nitrobenzoate, 49 mg (0.26 mmol) of iodine Cuprous chloride, 700 mg (5.1 mmol) potassium carbonate and 10 mL dry toluene. To the resulting suspension was added 0.055 mL (0.51 mmol) of N,N'-dimethylethylenediamine, and the mixture was heated to reflux for 16 hours. The mixture was then cooled to room temperature, then diluted with 100 mL of ethyl acetate, washed sequentially with 1N aqueous hydrochloric acid, saturated aqueous sodium bicarbonate, and brine (100 mL each), dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. Purification by fl...
Embodiment 2
[0253]
[0254] (2R,3R)-8-(methylsulfonyl)-3-(2,4,5-trifluorophenyl)-1,2,3,4-tetrahydropyrido-[1,2-a] Benzimidazol-2-amine bis(trifluoroacetic acid) salt
[0255] Step A: 2-Bromo-4-(methylthio)aniline
[0256] To a solution of 1.0 ml (8.0 mmol) 4-(methylthio)aniline in 40 ml of a 3:1 acetonitrile / carbon tetrachloride mixture was added 1.6 g (8.8 mmol) of N-bromosuccinimide. The reaction mixture was heated at reflux for 30 minutes, then cooled to room temperature and concentrated in vacuo. To the residue was added 100 mL of ethyl acetate and the organic solution was washed sequentially with saturated aqueous sodium bicarbonate and saturated brine (100 mL each), dried over sodium sulfate, filtered and evaporated in vacuo to give a viscous oil. The crude product was purified by flash chromatography (silica gel, 0 to 30% ethyl acetate / hexanes gradient) on a Biotage Horizon(R) system to afford the title compound. 1 H NMR (CDCl 3 ): δ7.45(s, 1H), 7.15(dd, J=8.3, 2.0Hz, 1H), 6...
Embodiment 29
[0269]
[0270] (7R,8R)-1-Chloro-7-(2,4,5-trifluorophenyl)-6,7,8,9-tetrahydropyrido[4',3':4,5]imidazo [1,2-a]pyridin-8-amine tris(trifluoroacetic acid) salt
[0271] Step A: [(7R,8R)-2-Epoxy-7-(2,4,5-trifluorophenyl)-6,7,8,9-tetrahydropyrido[4',3':4 ,5] tert-butyl imidazo[1,2-a]pyridin-8-yl]carbamate
[0272] To a solution of 48 mg of the Boc-protected intermediate of Example 27 in 2 mL of acetone was added m-chloroperbenzoic acid (28 mg). After stirring for 2 hours, the reaction mixture was concentrated and the residue was purified by reverse phase HPLC (YMC Pro-C18 column, gradient elution, 0% to 65% acetonitrile / water with 0.1% TFA). The residue was dissolved in ethyl acetate (10 mL), washed sequentially with saturated aqueous sodium bicarbonate (5 mL) and brine (5 mL), dried over anhydrous sodium sulfate, filtered and concentrated. LC / MS 435.1 (M+1).
[0273] Step B: [(7R,8R)-1-Chloro-7-(2,4,5-trifluorophenyl)-6,7,8,9-tetrahydropyrido[4',3':4, 5] tert-butyl imidazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com