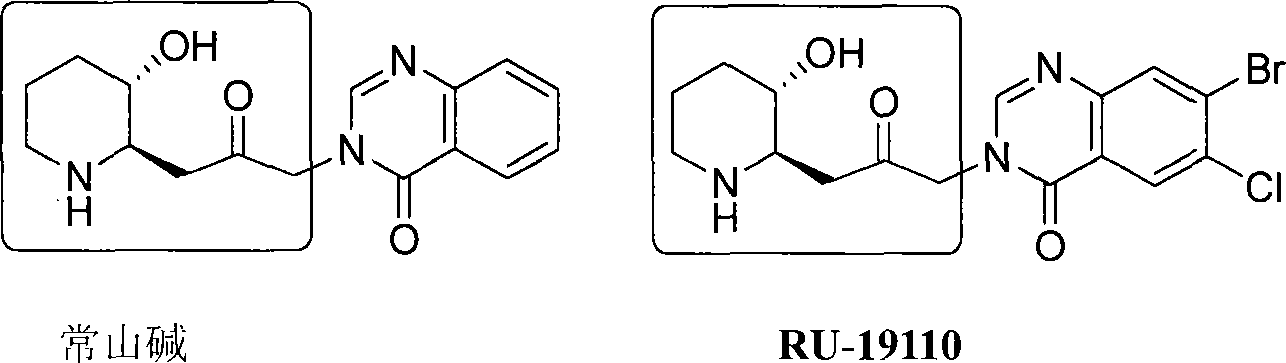
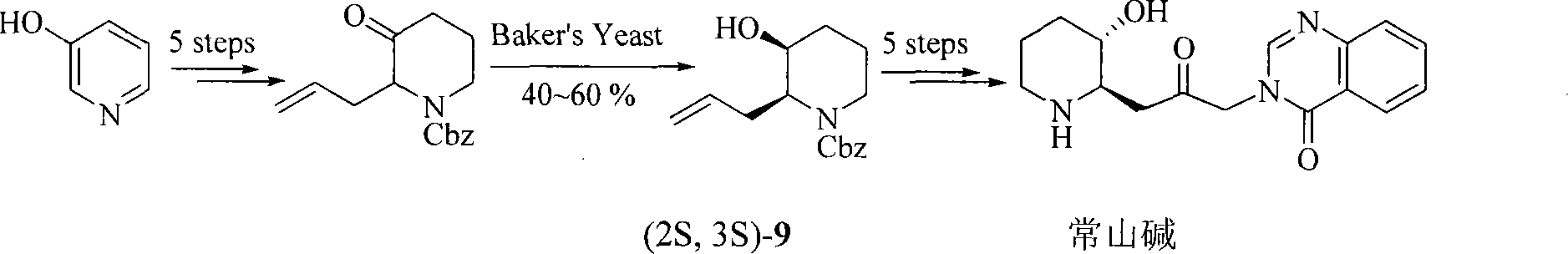
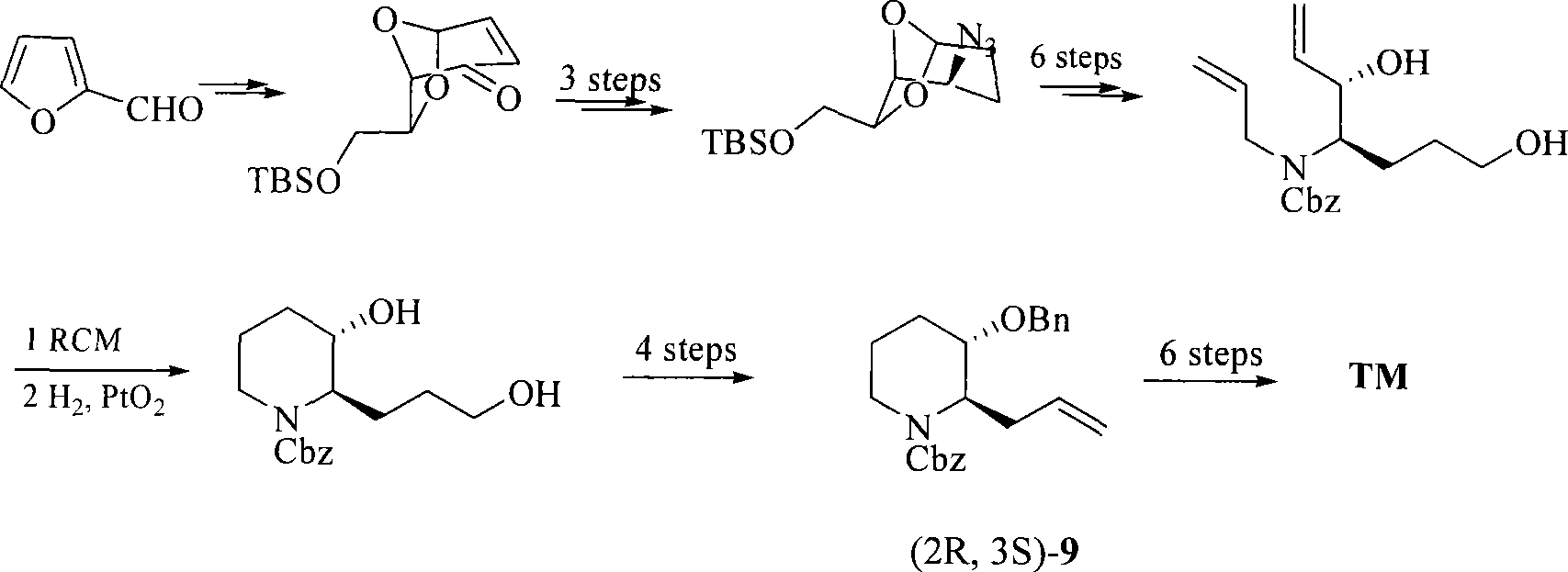
Process of synthesizing (2R, 3S)-3-alkyl siloxy-2-allyl-1-alkoxy carbonyl-pyridine
A technology of alkoxycarbonyl and alkylsiloxy, which is applied in the field of chemistry, can solve the problem of unsatisfactory selectivity of synthetic routes, and achieve the effects of high yield, low synthesis cost, and simple operation and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1: Compound 2——Synthesis of (S)-2-amino-5-(benzyloxy)carboxy-pentanoic acid
[0054] Under nitrogen protection, compound 1——L-glutamic acid (33.8mmol) was dissolved in concentrated sulfuric acid (150mL), benzyl alcohol (33.8mmol) was added, and the reaction was continued at 0-100°C for 2-10 hours. It can be obtained after extraction, drying, concentration and silica gel column chromatography. The reaction system was diluted with water, extracted three times with ethyl acetate, the organic layer was washed three times with saturated brine, anhydrous Na 2 SO 4 It was dried and concentrated to obtain compound 2 (yield 50.0%).
[0055] 2: Compound 3——Synthesis of (S)-2-hydroxy-5-(benzyloxy)carboxy-pentanoic acid
[0056] Compound 2 (20.7mmol) was dissolved in 150mL of anhydrous acetic acid, sodium nitrite (24.8mmol) was added, and the reaction was continued at 0°C to rt. for 2 to 10 hours. After concentration, it was diluted with water and extracted three times with ethy...
Embodiment 2
[0080] The synthesis of compounds 2-4 in steps 1-3 is the same as in Example 1.
[0081] Step 4, Compound 5——Synthesis of (S)-2-tert-butyldiphenylsilyloxy-5-(benzyloxy)carboxy-valeric acid methyl ester
[0082] Compound 4 (3.76 mmol) was dissolved in DMF (50 mL), tert-butyldiphenylchlorosilane (5.64 mmol) was added at rt., and stirring was continued for 5-20 hours. Diluted with water, separated, extracted with ethyl acetate, washed the organic phase three times with saturated sodium carbonate, washed with Na 2 SO 4 After drying and concentration, the crude product was purified by silica gel column chromatography to obtain compound 5 (yield 88.3%).
[0083] The synthesis of compounds 6-7 in steps 5-6 is the same as in Example 1.
[0084] Step 7, Compound 8——Synthesis of (S)-2-tert-butyldiphenylsilyloxy-5-(methanesulfonyl)oxy-pentanoic acid methyl ester
[0085] Compound 7 (1.86mmol) was dissolved in CH 2 Cl 2 (30mL), add p-methanesulfonyl chloride (2.05mmol) at 0°C, Et 3...
Embodiment 3
[0092] The synthesis of compounds 2-10 in steps 1-9 is the same as that described in Example 1.
[0093] Step 10, Compound 11——Synthesis of (S)-3-tert-butyldimethylsilyloxy-1-tert-butoxycarbonyl-2-piperidone
[0094] Compound 10 (2.27mmol) was dissolved in CH 2 Cl 2 (50mL), add (Boc) at 0°C 2 O (3.75mmol) and Et 3 N (4.54mmol), continuous reaction for 2-10 hours. Saturated NaHCO 3 The reaction was quenched, separated, the aqueous phase was extracted three times with dichloromethane, the organic phase was washed three times with saturated sodium chloride, washed with Na 2 SO 4 After drying and concentration, the crude product was purified by silica gel column chromatography to obtain compound 11 (yield 80.4%).
[0095] The synthesis of compounds 12-14 in steps 11-13 was the same as that described in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com