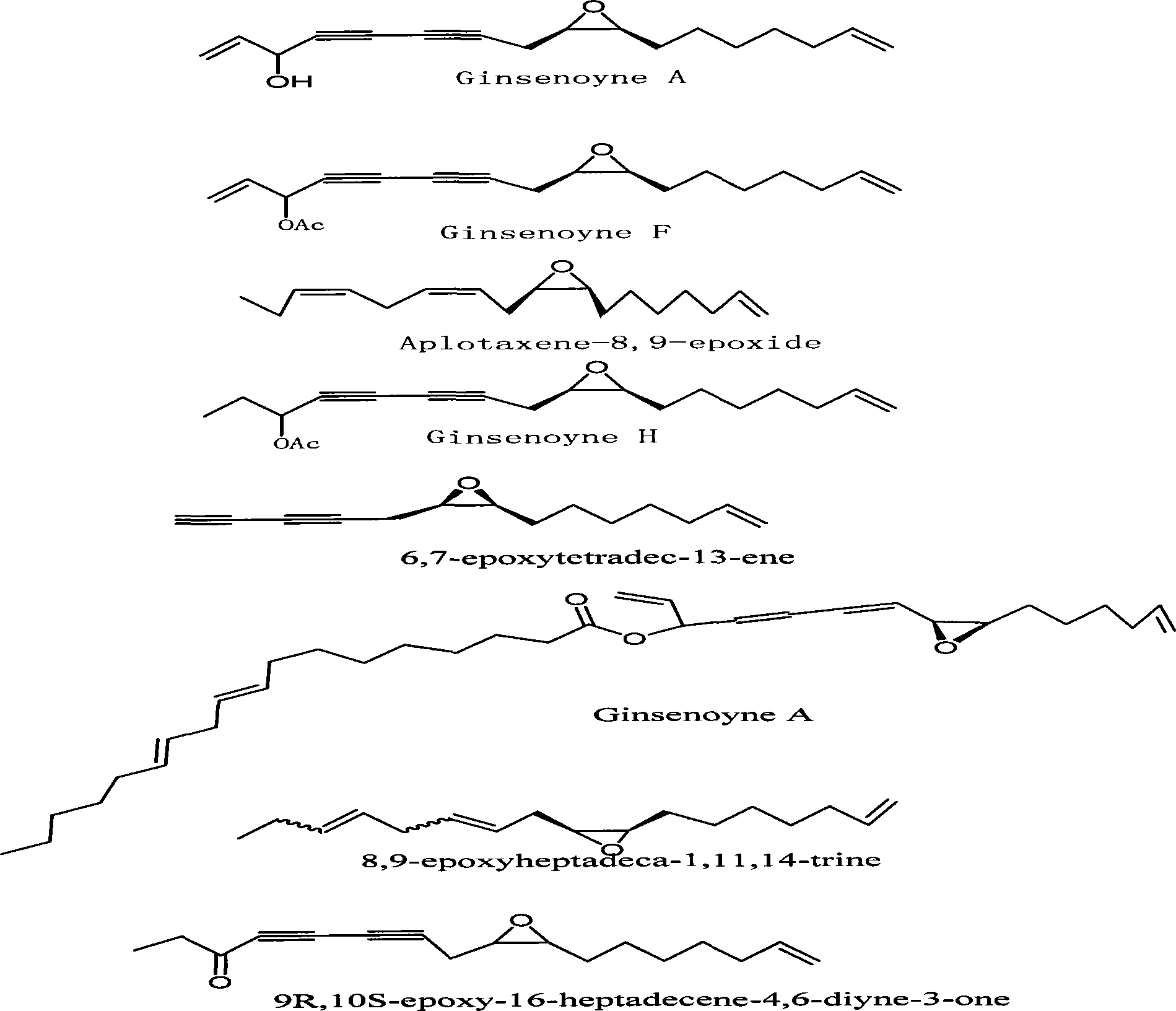
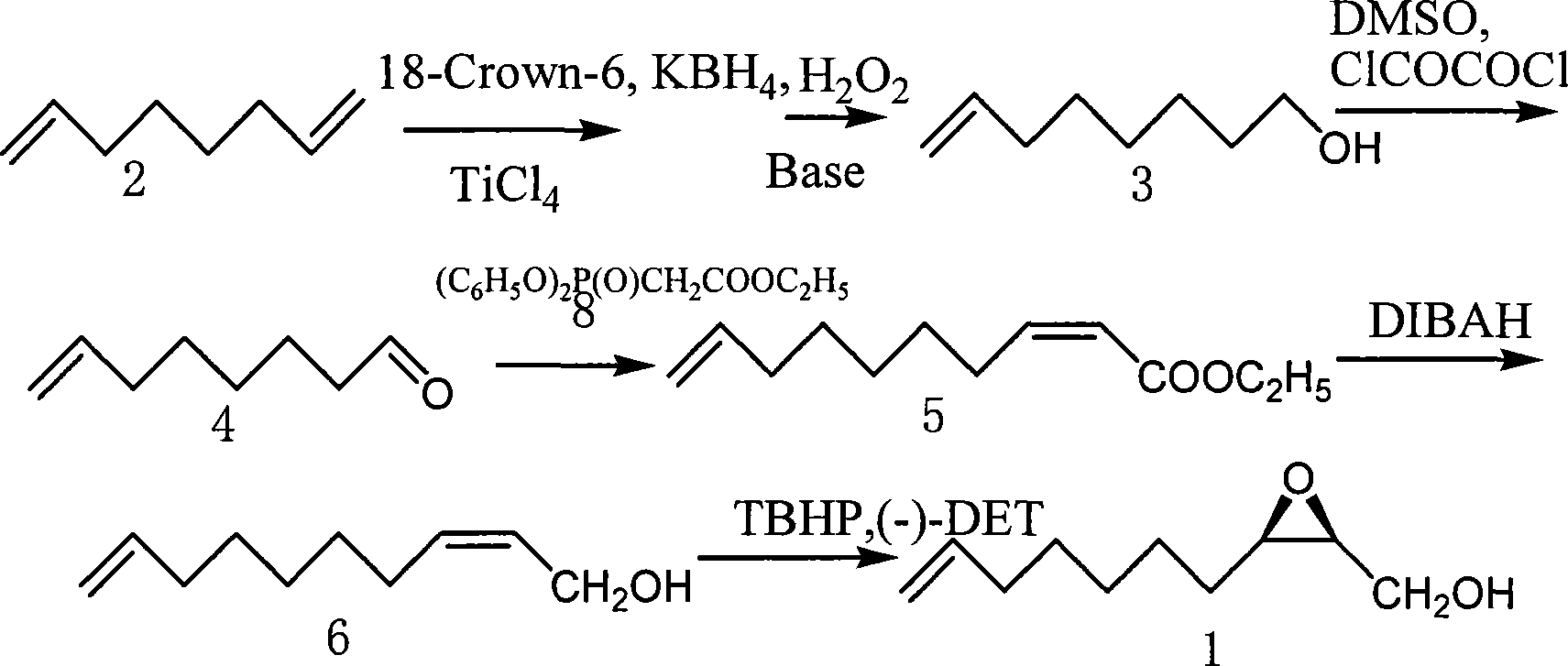
Fully-synthesizing method for (2R,3S)-epoxy-9-(aprylene-1-OL
A total synthesis and octene technology, applied in organic chemistry and other fields, can solve problems such as difficult to obtain a large amount of clinical research, complex and cumbersome extraction process, etc., and achieve the effect of high yield and simple separation and purification operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) In a 500ml three-necked flask with electric stirring, add 2.84g (50mmol) potassium borohydride (reducing agent), 0.264g (1mmol) 18-crown-6 (phase transfer catalyst), and 150ml tetrahydrofuran respectively under stirring, Cool to 5°C, slowly add 0.113ml (1mmol) TiCl dissolved in 10ml tetrahydrofuran dropwise 4 (titanium reagent, titanium tetrachloride), and then heated up to 30° C., and reacted for 0.5 hours to obtain a titanium composite (catalyst composite). Then 7.76ml (50mmol) of 1,7-octadiene (compound 2) was added to the above reaction system, and the reaction was continued for 4 hours to obtain an organoboron compound. Then the above reaction system was cooled to 5°C, 60ml of 6mol / L sodium methoxide and 120ml of hydrogen peroxide (hydrogen peroxide) (30% mass, concentration about 8.8mol / L) were added, then the temperature was raised to 65°C, reacted for 0.5 hours, and then used Diethyl ether was extracted 3 times, 50ml each time, and concentrated to obtain the...
Embodiment 2
[0038] 1) In a 500ml three-necked flask with electric stirring, add 1.9g (50mmol) sodium borohydride (reducing agent), 0.264g (1mmol) 18-crown-6 (transfer catalyst) and 150ml tetrahydrofuran under stirring respectively, Cool to 5°C, slowly add 0.113ml (1mmol) TiCl dissolved in 10ml tetrahydrofuran dropwise 3 (titanium reagent, titanium trichloride), and then heated up to 30° C., and reacted for 0.5 hours to obtain a titanium composite (catalyst composite). Then 7.76ml (50mmol) of 1,7-octadiene (compound 2) was added to the above reaction system, and the reaction was continued for 4 hours to obtain an organoboron compound. Then the above reaction system was cooled to 5°C, 60ml of 6mol / L sodium methoxide and 120ml of hydrogen peroxide (hydrogen peroxide) (30% mass, concentration about 8.8mol / L) were added, then the temperature was raised to 65°C, reacted for 0.5 hours, and then used Diethyl ether was extracted 3 times, 50ml each time, and concentrated to obtain the crude produc...
Embodiment 3
[0044] 1) with embodiment 1
[0045] 2) In a 250ml three-necked flask, place a constant pressure dropping funnel, an electric stirrer and a thermometer respectively, add 80ml of dry dichloromethane, protect it under argon at -70°C, first add oxalyl chloride (8.16ml, 90mmol), and then use a constant pressure Add DMSO (dimethyl sulfoxide) (13.92ml, 180mmol) into the dropping funnel, dilute with 20ml of dichloromethane, add dropwise to the flask, the rate of addition should be that the reaction solution does not exceed -40°C, and react for 15min , then 7-octen-1-ol (compound 3) (8.68g, 67.81mmol) was diluted with 20ml of dichloromethane, and then added dropwise to the reaction solution. The rate of addition should not exceed -60°C. After reacting for 1h, add 60ml of triethylamine, stir at -78°C for 15min, the reaction solution gradually rises to room temperature, then add 60ml of water, separate the organic phase with a separatory funnel, and extract the aqueous phase with ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com