Light-sensitive luminous europium complex and light-sensitive ligand molecular and their synthesizing method
A europium complex and photosensitive technology, applied in the field of photosensitive luminescent europium complexes, can solve the problems of low yield and difficult product separation, and achieve high yield, high rare-earth ion fluorescence quantum yield, and large two-photon absorption cross-section. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The synthesis method of the organic photosensitive molecule of the present invention may specifically comprise the following steps:
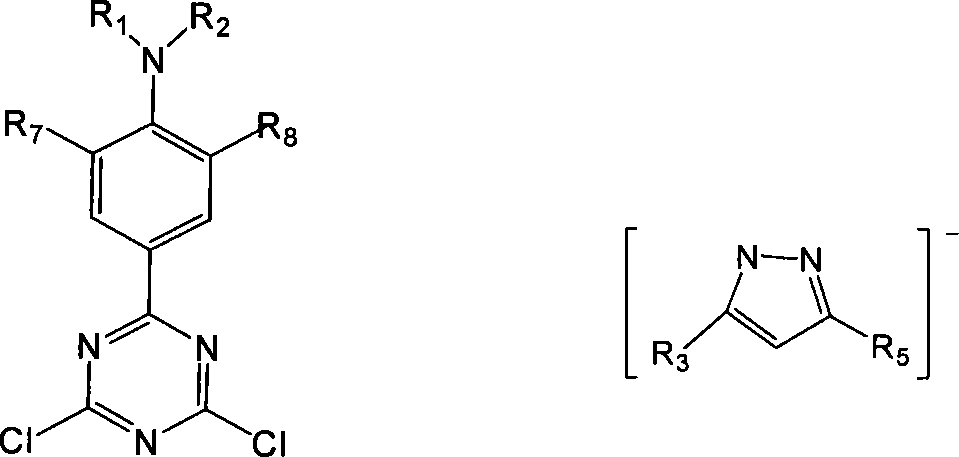
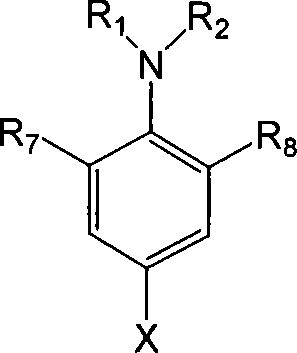
[0050] 1) P-N, N-dialkylamino-2,6-dimethylphenyllithium or p-N, N-dialkylamino-2-methylphenyllithium and tripolychlorazine (cyanide Uric acid chloride, cyanuric chloride) reaction, makes the substitution triazine compound shown in formula IV;
[0051] 2) reacting the substituted triazine compound obtained in step 1) with the pyrazole or substituted pyrazole anion shown in formula V to prepare the photosensitized organic ligand compound shown in formula I;
[0052] The synthetic method of europium complex of the present invention, its reaction process is described below
[0053]
[0054] Wherein, R in formula I, formula VI 1 , R 2 is an alkyl group with 1-4 carbon atoms, R 3 =R 4 =R 5 =R 6 is methyl or H, R 7 is methyl, R 8 is methyl or H.
Embodiment 1
[0055] Example 1, 2-(N,N-diethyl-2,6-dimethylanilino-4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)- 1,3,5-triazine (C 25 h 32 N 8 , formula VIII, that is, R in formula I 1 =R 2 =H 3 CH 2 C-, R 3 =R 4 =R 5 =R 6 =R 7 =R 8 =H 3 C-) Synthesis of Photosensitive Ligand Molecular Compound
[0056] Take 4 mmol of N,N-diethyl-2,6-dimethyl-p-bromoaniline, dissolve it in dried tetrahydrofuran, pass through argon to remove oxygen, place it in a dry ice acetone bath at -78°C, and Under protection, add 6.4mmol n-BuLi, slowly warm up to room temperature, continue stirring for 30min, add it to 5.4mmol tripolychlorazine frozen at -78°C, slowly warm up to room temperature, continue stirring for 60min. After removal of the solvent, the product was chromatographed on a silica gel column with dichloromethane as the eluent. The obtained crude product was recrystallized in petroleum ether to obtain 2-(N,N-diethyl-2,6-dimethylaniline-4-yl)-4,6-dichloro-1,3,5- Triazine yellow needle crystal ...
Embodiment 2
[0059] Embodiment 2, europium complex [Eu (tta) 3 VIII] (EuC 49 h44 N 8 f 9 o 6 S 3 )Synthesis
[0060] Under argon protection, 10mL Eu(tta) 3 ·3H 2 O (0.03 mmol) in tetrahydrofuran was added dropwise to 10 mL of the compound of formula VIII (0.03 mmol) prepared in Example 1 in tetrahydrofuran, and stirred at room temperature for 30 min. Remove the solvent, dissolve and filter with a small amount of ether, use n-hexane as a precipitant to precipitate a solid, wash it, and dry it to obtain 27 mg of a yellow powder.
[0061] The obtained yellow powder was subjected to mass spectrometry analysis, and the mass spectrometry (MALDI-TOF MS) characterization showed that the molecular ion peak M / Z=1260; elemental analysis (mass percentage): C, 46.80% (46.71%); H, 3.43% (3.52%); N, 9.01% (8.89%), is theoretical value in brackets; NMR analysis shows that product is [Eu(tta) 3 VIII], its structure is shown in formula XIV.
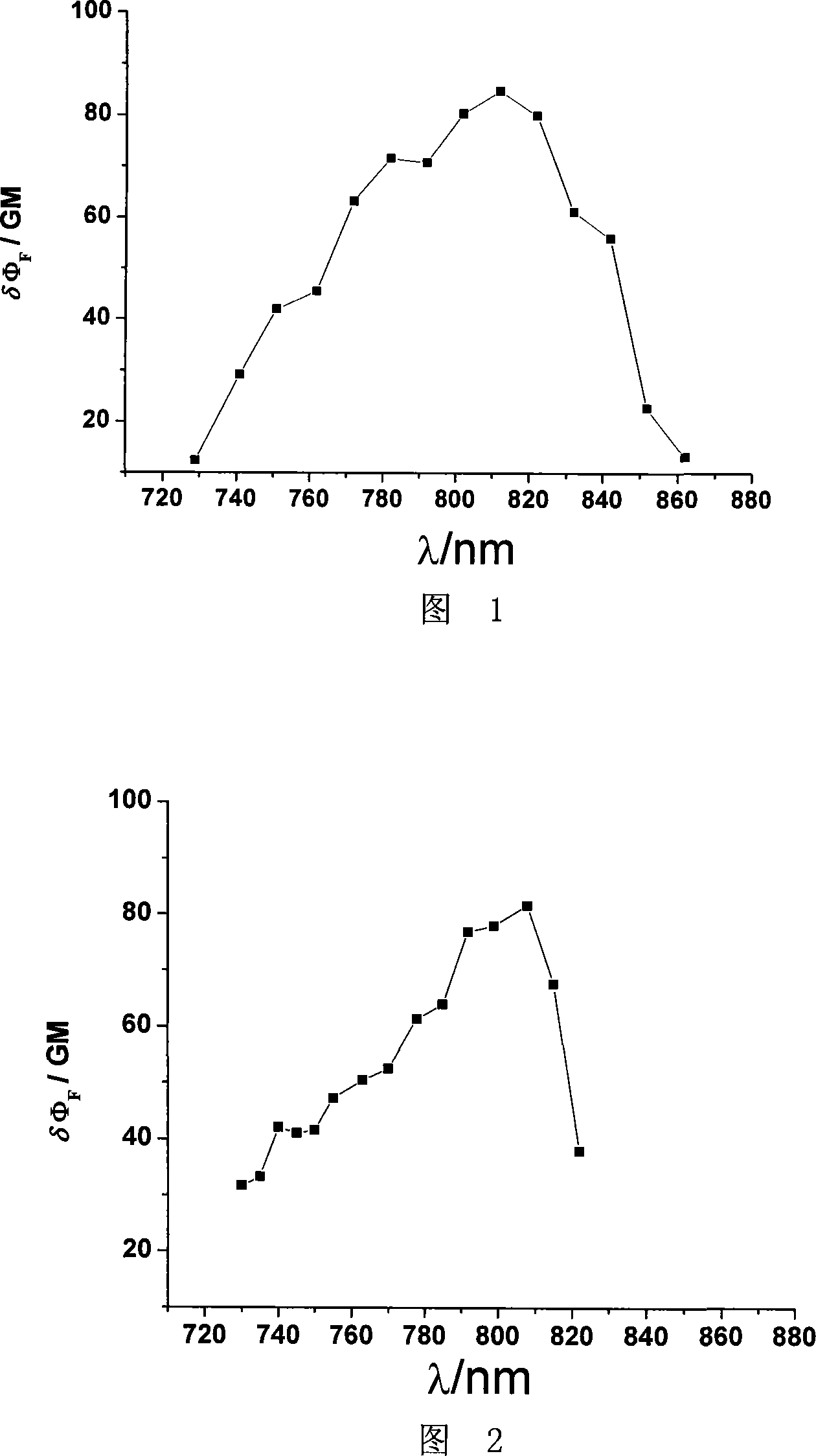
[0062] The complex [Eu(tta) 3 VIII] Dissolved in tolue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com