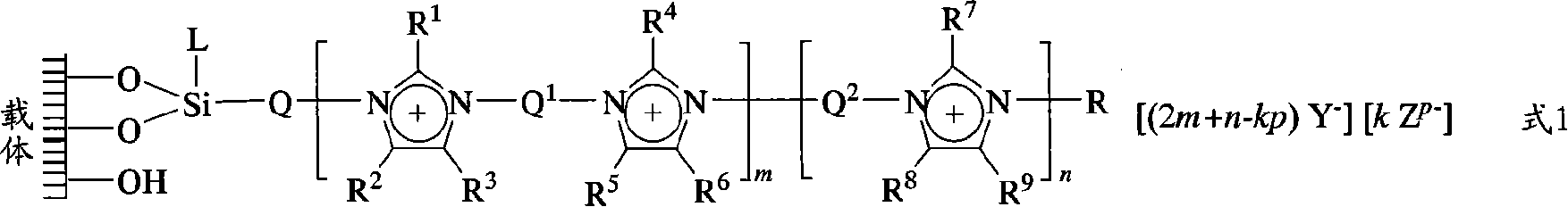
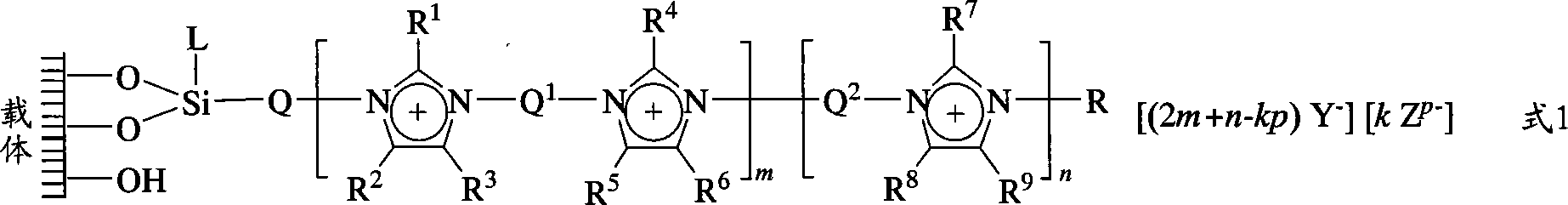
Solid-loaded ionic-liquid catalyst for olefin-dihydroxy reaction, its production and use
A technology of ionic liquid and catalyst, which is applied in the field of immobilized ionic liquid-loaded transition metal catalytic active center catalyst and its preparation, can solve the problems of complicated separation and purification, difficulty in catalyst recycling, high price, etc., and achieve the effect of simple product separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Take 8.00g (11.5mmol) peroxytungstate K 2 [{W(=O)(O 2 )(H 2 O)} 2 (μ-O)]·2H 2 O was dissolved in 100mL distilled water, and 15.0g of SiO 2 / tri-RTILs-CH 3 / PF 6 (For the synthesis method, see Chinese patent ZL.200710017755.2, the structure is shown in Formula 2) Suspend in the solution, and react with mechanical stirring at room temperature for 3 days. After the reaction, filter with suction, wash the solid with distilled water, and dry it in vacuum (50°C, -0.07MPa) to obtain powdered catalyst SiO 2 / tri-RTILs-CH 3 / W. Environmental scanning electron microscopy (SEM-EDAX) analysis of the surface of the catalyst revealed: C, 24.93; O, 26.59; Br, 0.53; Si, 38.45; W, 9.51wt%.
[0037] SiO 2 / tri-RTILs-CH 3 / PF 6
[0038] Formula 2
[0039] will be prepended with H 3 PO 4 1.135 g (10 mmol) of 30% H adjusted to pH = 1.5 2 o 2 , 1.64g (20mmol) freshly distilled cyclohexene, immobilized three-layer ionic liquid catalyst SiOx / tri-RTILs-CH 3 / W 1mol% mixed, ...
Embodiment 2-9
[0041] The composite catalysts used successively in the dihydroxylation reaction of embodiment 2-9 are four kinds of immobilized monolayer imidazole ionic liquid SiO 2 / mono-RTIL-R / W and four immobilized bilayer imidazolium ionic liquid SiO 2 / di-RTILs-R / W, their preparation method is the same as the composite catalyst preparation method of embodiment 1, by base catalyst SiO 2 / mono-RTIL-R / PF 6 (Equation 3) and SiO 2 / di-RTILs-R / PF 6 (Formula 4) and peroxytungstate K 2 [{W(=O)(O 2 )(H 2 O)} 2 (μ-O)]·2H 2 Formed by the action of O, the preparation method of the immobilized double-layer imidazole ionic liquid in these two types of basic catalysts can be found in Chinese patent ZL.
[0042] R=a)-C 4 h 9 b)-C 8 h 17 d)-C 6 h 5 CH 2 e)-C 12 h 25 SiO 2 / mono-RTIL-R / PF 6
[0043] Formula 3
[0044] R=a)-C 4 h 9 b)-C 8 h 17 d)-C 6 h 5 CH 2 e)-C 12 h 25 SiO 2 / di-RTILs-R / PF 6
[0045] Formula 4
[0046] implement
[0047...
Embodiment 10
[0049] 2.50g (10mmol) tungstic acid H 2 WO 4 Add to 7 mL 30% H in portions 2 o 2 The solution was stirred and reacted for 2 hours, the reaction mixture was suction filtered with a funnel, and 0.3 mL (1.25 mmol) of 80% H was added to the filtrate at room temperature. 3 PO 4 Solution, 5mL acetone, 1.00g immobilized three-layer ionic liquid SiO 2 / tri-RTILs-CH 3 / PF 6 (See Chinese patent ZL.200710017755.2 for the synthesis method, the structure is shown in Formula 2), and the reaction was stirred for 72 hours. Suction filtration, the solid was washed with distilled water, and dried in vacuum (50°C, -0.07MPa) to obtain powdered catalyst SiO 2 / tri-RTILs-CH 3 / PW (its anion is [PO 4 {W(O)(O 2 ) 2} 4 ] 3- form exists). Environmental scanning electron microscope energy spectrum (SEM-EDAX) analysis catalyst surface, measured: C, 20.17; O, 33.66; Si, 36.78; P, 0.46; W, 8.93wt%.
[0050] The dihydroxylation reaction of cyclohexene is by the method for embodiment 1, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com