Alpha-galactosidase gene, its coding protein, production and use
A galactosidase and coding technology, applied in the field of genes and their encoded proteins, can solve problems such as unsatisfactory decomposition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The separation and purification of embodiment 1 Penicillium α-galactosidase
[0063] Penicillium (Penicillium) F63 was inoculated in the enzyme-producing medium to induce the expression of α-galactosidase. After 7 days of induction, centrifuge the thalli to obtain the culture supernatant, and the culture supernatant was washed with 20-95% (NH 4 ) 2 SO 4 For precipitation, the pellet was resuspended in 20 mM Tris-HCl, pH 8.0, and dialyzed overnight.
[0064] Dialyzed samples were concentrated using ultrafiltration tubes. A 2 mL sample was loaded onto a HiTrap Q Sepharose XL anion column (Amersham Pharmacia Biotech) pre-equilibrated with 20 mM Tris-HCl, pH 8.0. The protein bound to the column was eluted with a NaCl salt gradient, and the protein containing the α-galactosidase component was eluted under a 0.3M salt gradient.
[0065] Sephacryl S-200 HR molecular sieves (Amersham Pharmacia Biotech) were applied to 500uL concentrated samples containing α-galactosidase c...
Embodiment 2
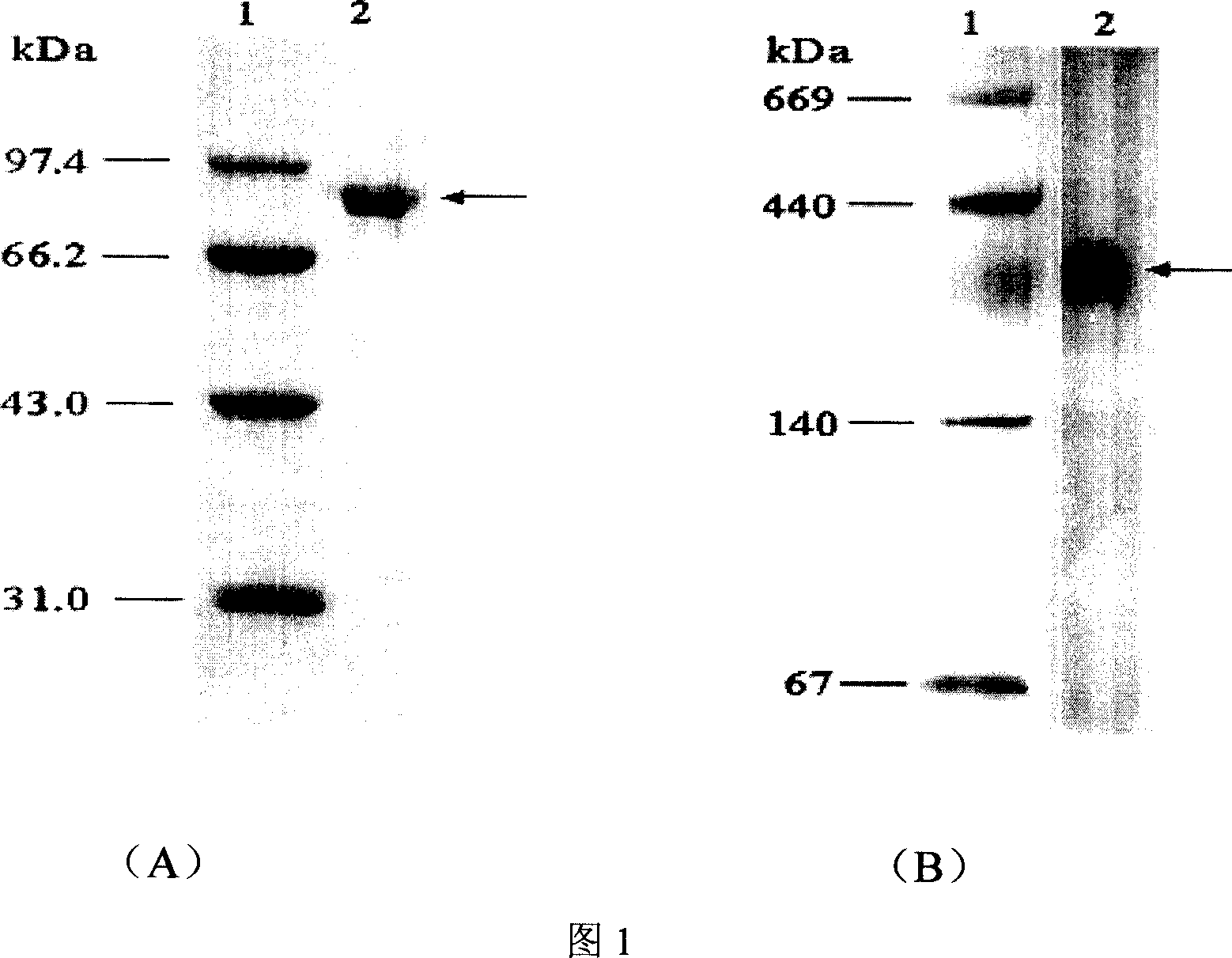
[0069] Example 2 Sequencing of α-galactosidase protein.
[0070] The α-galactosidase purified by molecular sieves in Example 1 was concentrated, subjected to SDS-PAGE electrophoresis, and a single band of electrophoretic pure α-galactosidase was subjected to in-gel digestion. The peptides were sequenced with a mass spectrometer to obtain 6 internal peptides. The amino acid sequences of these 6 internal peptides were LFVLDDGWFK, QSEGYTVSEFQYK, VNPLVLTGDMWR, DNAGLGDWLPNP, LEGLDENALYK and PEVQDFLLK, respectively. After sequence comparison, it was found that 4 of the endopeptides had high homology with known α-galactosidases, and no homologous α-galactosidase sequences were found for the other two endopeptides . Among them, the homology of LFVLDDGWFK and the corresponding peptides of Aspergillus niger, Aspergillus nidulans, Trichoderma, Absidia α-galactosidase is 100%, 88%, 100% and 88%; The homology of the corresponding peptides of Aspergillus and Trichoderma α-galactosidase is...
Embodiment 3
[0071] Example 3 Cloning of Penicillium α-galactosidase coding gene Agl1
[0072] Genomic DNA extraction of Penicillium sp (Penicillium sp) F63: take the Penicillium sp F63 bacterial liquid cultured at 30°C for 7 days and centrifuge at 60000rpm for 10min. Take 100mg of mycelia and add 500μL of sterile water to wash, centrifuge to get the precipitate.
[0073] The precipitate was resuspended in 500 μL extract mixture, incubated at 37°C for 60 min, and centrifuged at 10,000 rpm for 10 min to remove the precipitate. The supernatant was extracted sequentially with equal volumes of phenol, phenol:chloroform, and chloroform. Take the upper layer solution and add 0.6-1 times the volume of isopropanol to precipitate at room temperature for 10 minutes. Centrifuge at 12000rpm for 15min. The precipitate was washed with 70% ethanol, centrifuged slightly, dried and dissolved in 30 μL sterile water for later use.
[0074] According to the internal peptide sequence of sequenced α-galacto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com