Method for synthesizing alpha-pyranone derivatives
A synthesis method and derivative technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions, large environmental pollution, and large reaction waste liquid, and achieve the effects of mild reaction conditions, less environmental pollution, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
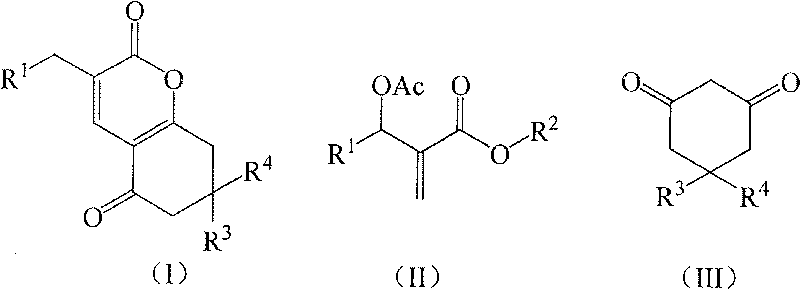
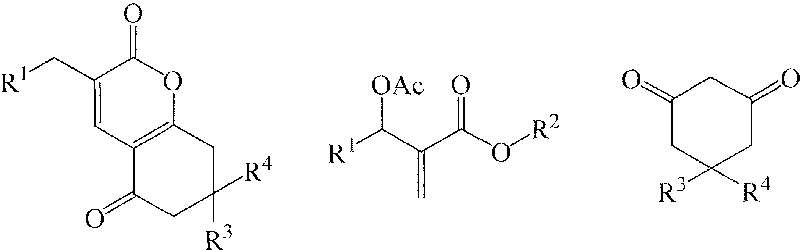
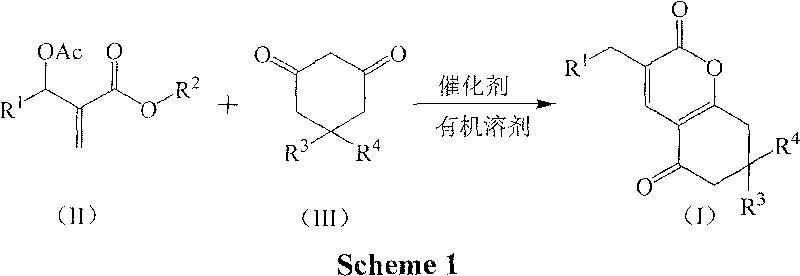
[0018] Preparation of Example 13-(3-nitrobenzyl)-7,8-dihydro-6H-chromene-2,5-dione (1a)
[0019] In a 250mL three-necked flask equipped with a thermometer, a reflux condenser, and mechanical stirring, 11.16 g (40 mmol) of 2-(acetoxy-(3-nitrophenyl))methyl methacrylate, 1,3-cyclo 4.48g (40mmol) of hexanedione, 2.76g (20mmol) of potassium carbonate, and 33.48g of acetone were stirred and reacted at 60°C for 4 hours. TLC was followed until the reaction of the raw materials was complete, then saturated saline was added, and extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure. The crude product was recrystallized from petroleum ether to obtain 9.39 g of 3-(3-nitrobenzyl)-7,8-dihydro-6H-chromene-2,5 - Diketone, pale yellow crystal, the yield is 78.5%, the melting point is 144.3-144.7°C, and the HPLC purity is 98.7%. 1 H NMR (CDCl 3 , 400MHz) δ8.11(m, 2H), 7.63(m, 2H), 7.49(t, 2H, J=7.6Hz), ...
Embodiment 2
[0020] Preparation of Example 23-(3-nitrobenzyl)-7,8-dihydro-6H-chromene-2,5-dione (1a)
[0021] The molar ratio of the feed material is Baylis-Hillman adduct: 1,3-cyclohexanedione: base is 1.0: 1.0: 1.0, wherein the Baylis-Hillman adduct is 2-(acetoxy-(3-nitro Phenyl)) methyl methacrylate, the base is potassium carbonate, and the organic solvent is acetone, and its consumption is 5 times that of 2-(acetoxy-(3-nitrophenyl)) methyl methacrylate.
[0022] Others are with embodiment 1. The product is 3-(3-nitrobenzyl)-7,8-dihydro-6H-chromene-2,5-dione, the yield is 82.2%, the melting point is 143.9-144.5°C, and the HPLC purity is 98.5%.
Embodiment 3
[0023] Preparation of Example 33-(3-nitrobenzyl)-7,8-dihydro-6H-chromene-2,5-dione (1a)
[0024]The molar ratio of the feed material is Baylis-Hillman adduct: 1,3-cyclohexanedione: base is 1.0: 1.0: 2.0, wherein the Baylis-Hillman adduct is 2-(acetoxy-(3-nitro Phenyl)) methyl methacrylate, the base is N, N-dimethylaniline, the organic solvent is 1,2-dichloroethane, and its consumption is 2-(acetoxy-(3-nitrophenyl )) 8 times that of methyl methacrylate, stirred and reacted at 60°C for 2 hours.
[0025] Others are with embodiment 1. The product is 3-(3-nitrobenzyl)-7,8-dihydro-6H-chromene-2,5-dione, the yield is 82.6%, the melting point is 144.5-144.8°C, and the HPLC purity is 98.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com