Process and apparatus for treating waste water containing metal complex
A technology for complexing metal wastewater and wastewater, applied in water/sewage treatment, metallurgical wastewater treatment, chemical instruments and methods, etc., can solve the problems that ion exchange cannot completely remove complexing agents, low cost, and good treatment effect. Achieve strong catalytic activity and catalytic effect, prevent agglomeration or passivation, and achieve emission standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Iron powder reduction-Fenton oxidation synergistic treatment of EDTA complexed copper wastewater
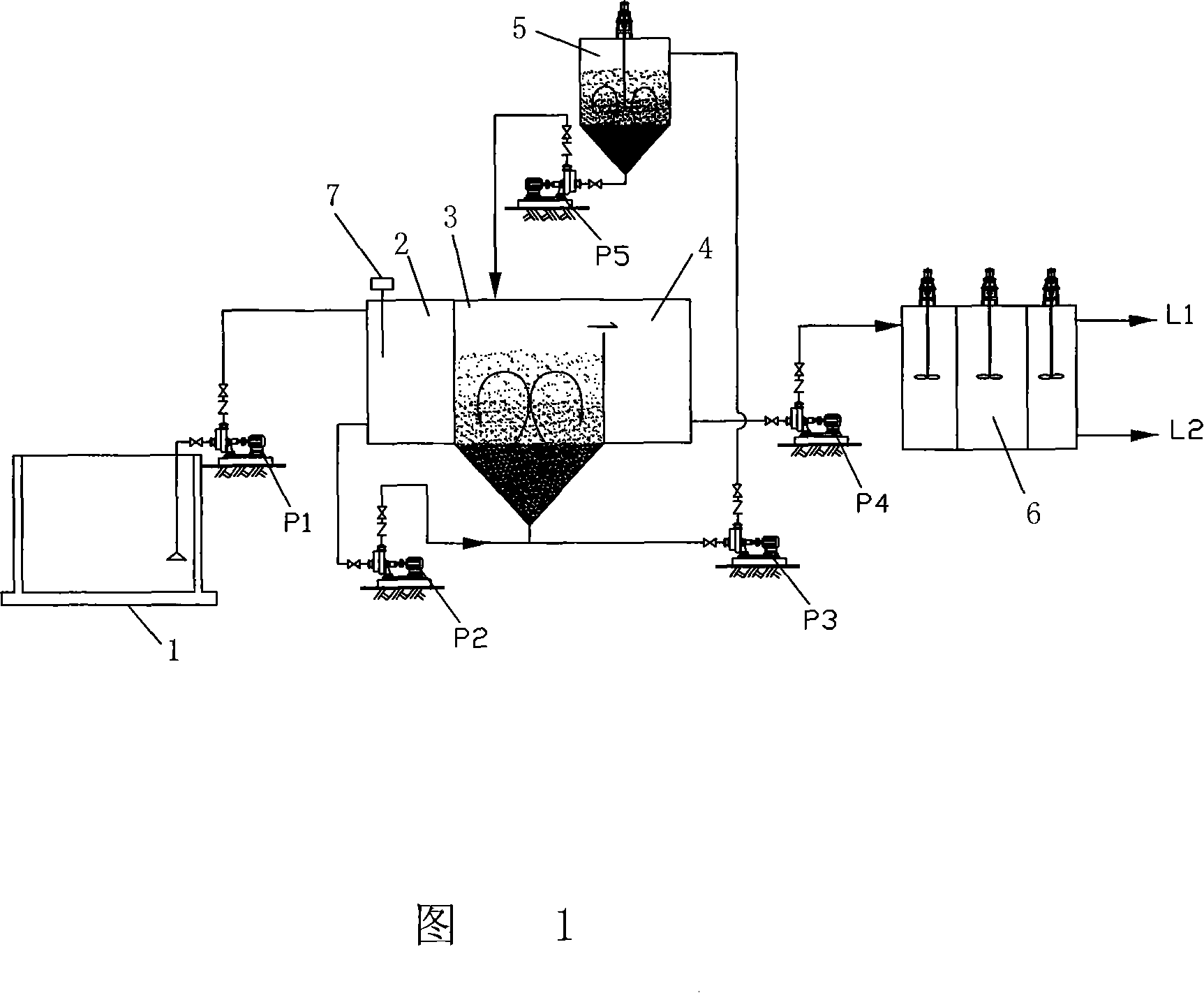
[0029] Referring to Fig. 1, it includes a water collecting tank 1, a pH regulating tank 2, a fluidized bed decomplexing reactor 3, a regulating tank 4, a regeneration bed 5, and a coagulation reaction sedimentation tank 6; P1 imports the wastewater from the sump 1 to the pH adjustment tank 2; by adding acid-base chemicals, the pH value in the pH adjustment tank 2 is adjusted, and the pH meter 7 in the pH adjustment tank 2 can accurately control the pH of the wastewater in the pH adjustment tank 2 value, and at the same time put oxidant H in the regulating pool 2 o 2 ; The pH adjustment tank 2 is connected to the fluidized bed decomposition reactor 3 through a pipeline, and the waste water is input from the regulating tank to the fluidized bed decomposition reactor 3 by the pump P2 on the pipeline; the top of the fluidized bed decomposition reactor 3 It is conne...
Embodiment 2
[0035] Example 2 Iron powder reduction-Fenton oxidation synergistic treatment of citric acid complex copper wastewater
[0036] According to the equipment and process of Example 1, the pH of 250ml of citric acid complex copper wastewater with a concentration of 5mg / l (calibrated with copper ions) was adjusted to 2.5 (initial pH was 3.2, adjusted with hydrochloric acid, and accurately controlled by a pH meter ), and follow H 2 O 2 :COD=2.0:1 (use H for each COD value 2 O 2 2 mg) to the wastewater by adding H 2 O 2 (The COD value of the solution was measured by a COD rapid measuring instrument), and the flow rate was 10m / h from the bottom of the fluidized bed breaking reactor. Due to the impact of the liquid flow, the iron powder with a particle size of 0.5mm ( excess) in a fluidized state.
[0037] In the fluidized bed breaking reactor, the iron powder in the fluidized state is fully contacted with the free state metal ions in the wastewater, and a displacement reaction ...
Embodiment 3
[0041] Example 3 Iron powder reduction-Fenton oxidation synergistic treatment of wastewater containing direct copper complex azo dyes
[0042] According to the equipment and process of Example 1, adjust the pH of 100 ml of wastewater containing direct copper complex azo dyes (initial pH value is 7.01; COD is 110.9 mg / L; chromaticity, that is, absorbance, is 0.144) to 4 (using Hydrochloric acid regulation, precise control by pH meter), and according to H 2 O 2 :COD=1.4:1 (use H for each COD value 2 O 2 1.4 mg) to the wastewater by adding H 2 O 2 . The flow rate is 12m / h from the bottom of the fluidized bed breaking reactor. Due to the impact of the liquid flow, the iron powder (excess) with a particle size of 0.5mm filled in the bed is in a fluidized state.
[0043] In the fluidized bed breaking reactor, the iron powder in the fluidized state is fully contacted with the free state metal ions in the wastewater, and a displacement reaction occurs. When the free copper ion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com