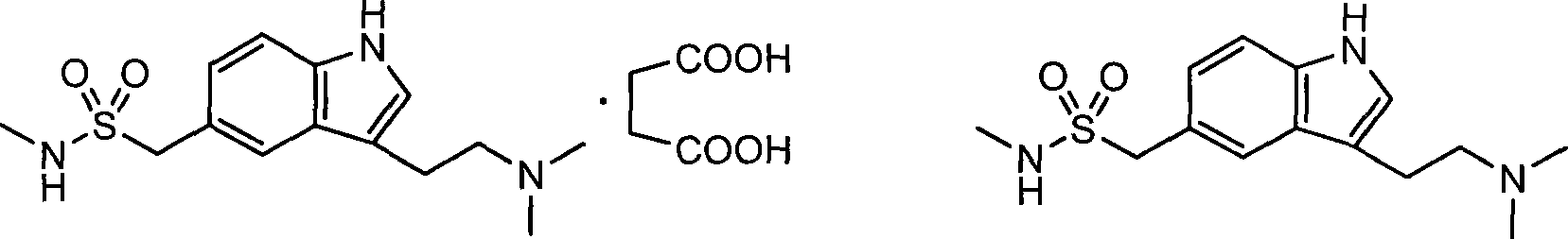Method for preparing Sumatriptan Succinate
A technology for sumatriptan succinate and hydrochloride, which is applied in the field of preparation of sumatriptan succinate, can solve problems such as difficult to realize industrialization requirements, unsuitable for industrialization, harsh reaction conditions, etc., achieve shortened production cycle, easy The effect of simple purification and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
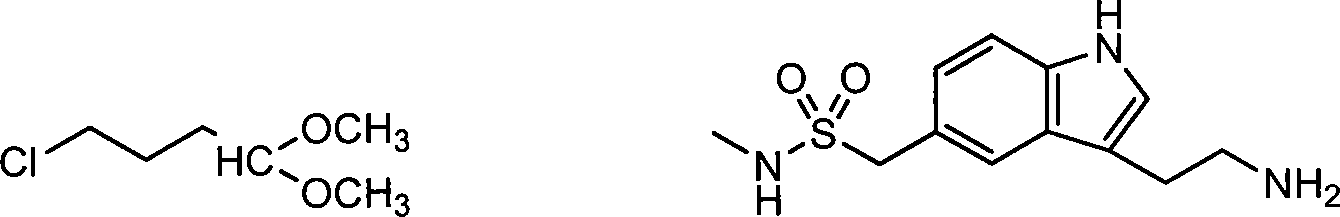
[0039] Add 106.8g (1.0mol) of 4-chlorobutyraldehyde (GC content 99.7%, commercially available) into a 500ml three-necked flask, stir and cool, and start to drop 209.1g (1.1mol) of sodium metabisulfite and 315ml of water at 20°C The prepared solution. After the dropwise addition, stir at room temperature for 0.5 hour, filter, and dry to obtain 289.2 g (content 98.6%) of white solid 4-chlorobutane-1, 1-sodium disulfonate with the structure shown in general formula IX, yield 96.1% .
Embodiment 2
[0041] 106.8g (1.0mol) 4-chlorobutyraldehyde (GC content 99.7%, commercially available) was joined in the there-necked flask of 500ml, stirred, and began to dropwise add 190.1g (1.0mol) of sodium metabisulfite and 290ml of water at room temperature. solution. After the dropwise addition, stir at room temperature for 0.75 hours, filter, and dry to obtain 288.9 g (content 98.4%) of white solid 4-chlorobutane-1,1-sodium disulfonate with the structure shown in general formula IX, yield 95.8% .
Embodiment 3
[0043]62.8g (208.6mmol) of 4-chlorobutane-1,1-disulfonic acid prepared in 50.0g (198.7mmol) of 4-hydrazine-N-methylbenzenesulfonamide hydrochloride and embodiment 1 Sodium was added to a 1L three-necked flask, and then 550ml of 5% sulfuric acid was added. Heat to 65±2°C and hold for 2 hours. After the incubation is completed, adjust the pH of the reaction solution to neutral with 25% aqueous sodium hydroxide solution. Extracted 3 times with ethyl acetate, combined the organic phases and washed 3 times with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, distilled and recovered ethyl acetate, and the residue was about 35.0g oil, which was 3-(2-chloro The crude product of ethyl)-N-methyl-1H-indole-5-methanesulfonamide (structure shown in general formula X) has a content of 93.4% (HPLC), and a yield of 57.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com