Application of mannose in the preparation of medicine for treating pulmonary inflammation disease
A technology of mannose and medicine is applied in the application field of mannose in the preparation of medicines for treating pulmonary inflammatory diseases, which can solve the problems of limited treatment means, adverse effects, adverse reactions and the like, and achieves low price, strong practicability, and ease of use. The effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
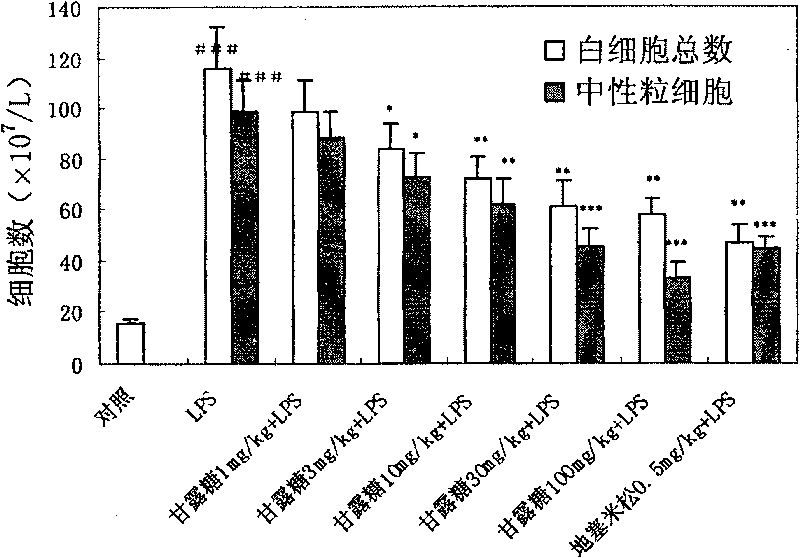
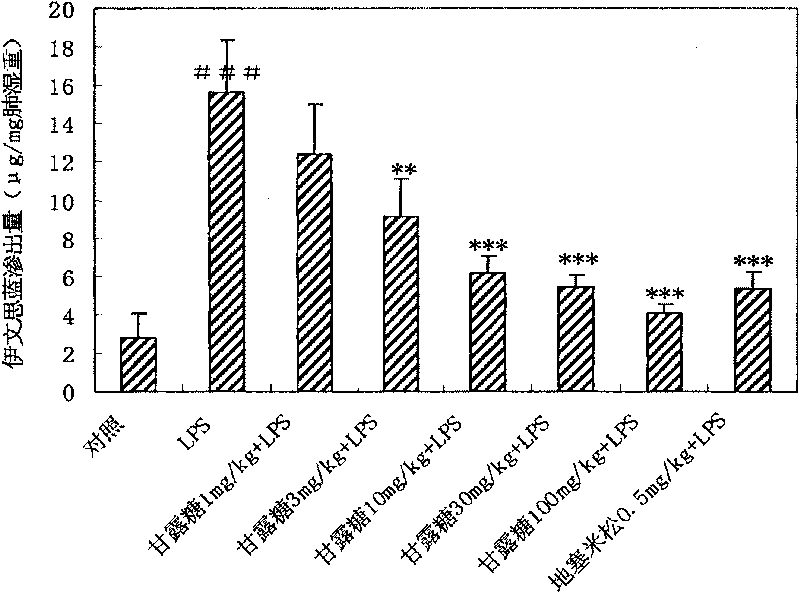
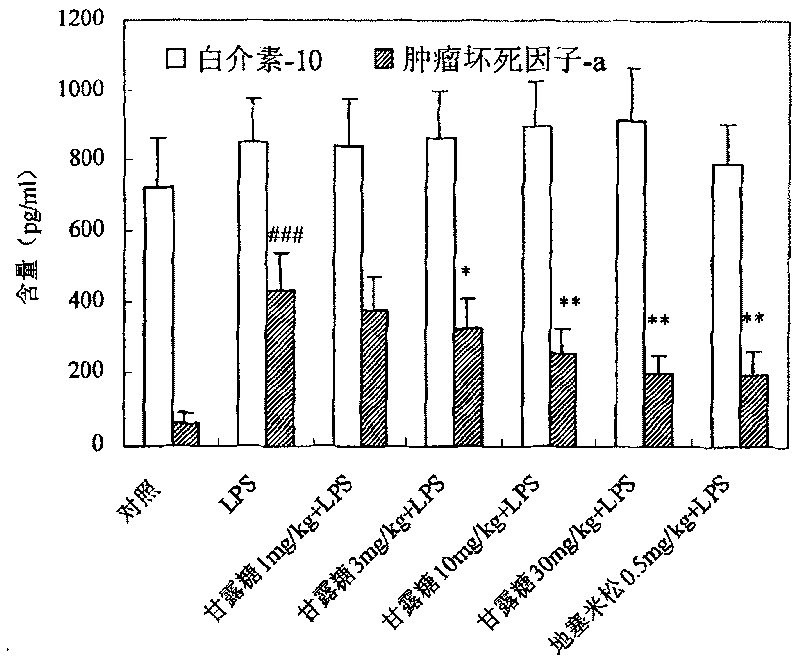
[0034] Example 1 Protective effect of mannose on acute lung injury in mice
[0035] Experimental Materials and Methods
[0036] 1. Experimental animals
[0037] Clean grade (Grade II) male ICR strain mice (weight 20-22 g) were provided by the Experimental Animal Center of Zhejiang University School of Medicine, certificate number: SYXK (Zhe) 20040052. All operations and experimental procedures abide by the Regulations on the Administration of Laboratory Animals. Animals had free access to food and water before the experiment.
[0038] 2. Main reagents and instruments
[0039] (1) Lipopolysaccharide (LPS, Esherichia coli O127:B8): American Sigma Company;
[0040] (2) Mannose (D-mannose): American Sigma Company;
[0041] (3) Dexamethasone Sodium Phosphate Injection (dexamethasone, DXM, batch number: 050502): Zhejiang Xianju Pharmaceutical Co., Ltd.;
[0042] (4) Chloral hydrate: domestic analytically pure product;
[0043] (5) Urethane: domestic analytical pure product; ...
Embodiment 2
[0107] Example 2 The protective effect of mannose on the rat model of acute respiratory distress syndrome
[0108] The medicine prepared from the mannose of the present invention is at the doses of 15 mg / kg, 45 mg / kg, 135 and 405 mg / kg, and is controlled by other monosaccharides such as glucose 135 mg / kg, galactose 135 mg / kg and fructose 135 mg / kg , intravenous administration, prevention and treatment of endotoxin lipopolysaccharide (LPS)-induced acute respiratory distress syndrome model in rats, the results show that: mannose can protect LPS-induced arterial oxygen partial pressure decrease, arterial carbon dioxide partial pressure increase, pulmonary vascular ventilation Increased permeability, pulmonary edema, accumulation of inflammatory cells in the lungs, increased cytokine TNF-α, increased levels of myeloperoxidase (MPO), and decreased levels of superoxide dismutase (SOD). But other monosaccharides such as glucose 135mg / kg, galactose 135mg / kg and fructose 135mg / kg have ...
Embodiment 3
[0167] The prevention and treatment of embodiment 3 mannose to severe acute respiratory syndrome (SARS)
[0168]In mid-November 2002, the first case of infectious atypical pneumonia of unknown etiology (referred to as SARS) was discovered in Guangdong Province of my country. The disease quickly spread in Hong Kong and many other countries and regions, and almost all people who had close contact with patients with the disease were infected, with the highest infection rate among healthcare workers. Therefore, it immediately attracted the attention of the people, governments and the World Health Organization all over the world, and researchers from various countries also quickly joined the research on SARS (Zhao Xirong, et al. Clinical and Pathological Research Progress of SARS. China Journal of Hospital Infection 2003, 11(3): 1092-94). In early March 2003, the World Health Organization (WHO) issued a warning to the world and named the disease Severe Acute Respiratory Syndrome (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com