Making method for nano LiFePO4-carbon composite cathode material
A technology of lithium iron phosphate and positive electrode materials, applied in the direction of electrode manufacturing, phosphorus compounds, chemical instruments and methods, etc., can solve the problems of difficult control of product purity, influence on rate performance, complicated preparation process, etc., and achieve excellent cycle stability , low price, simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
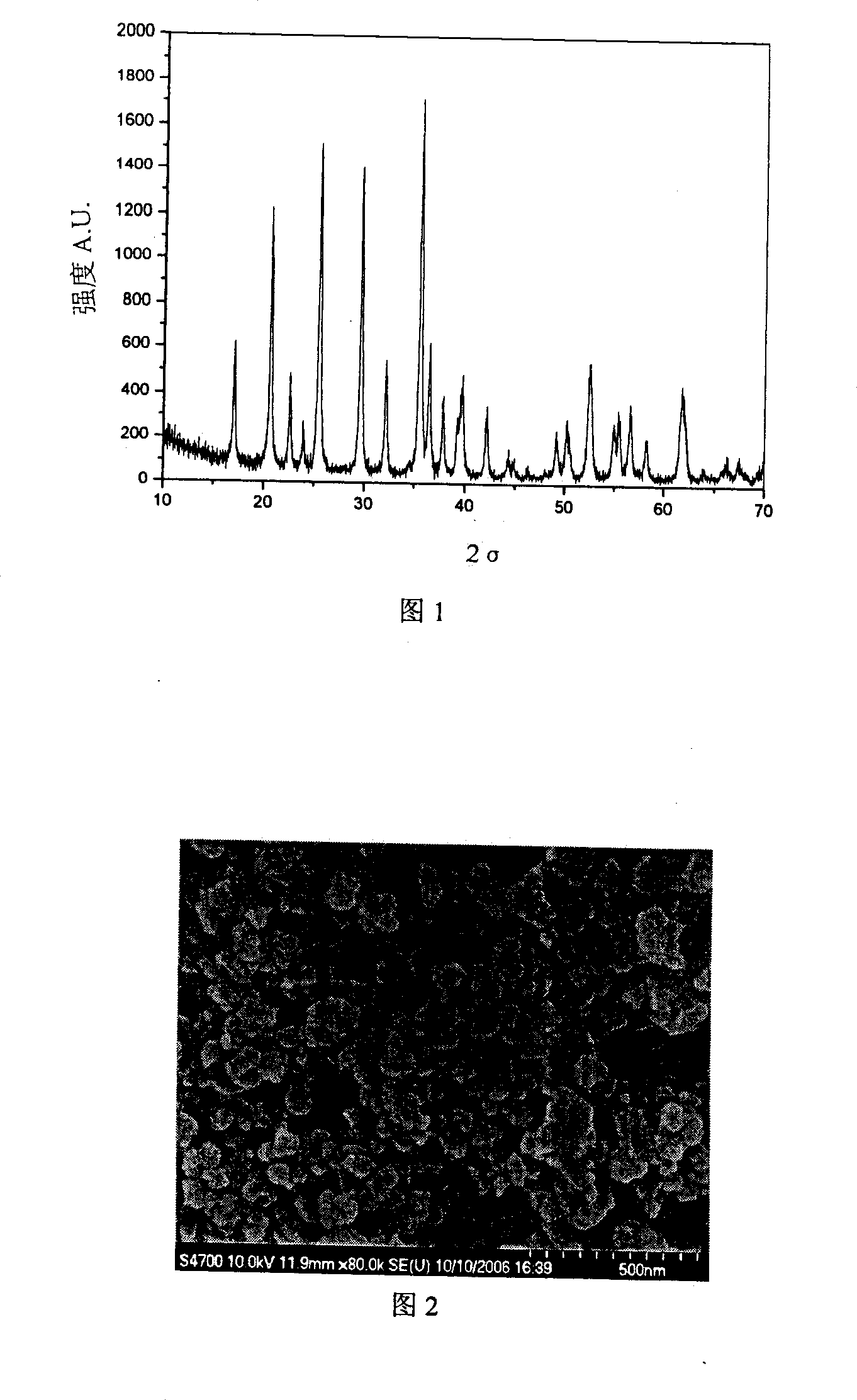
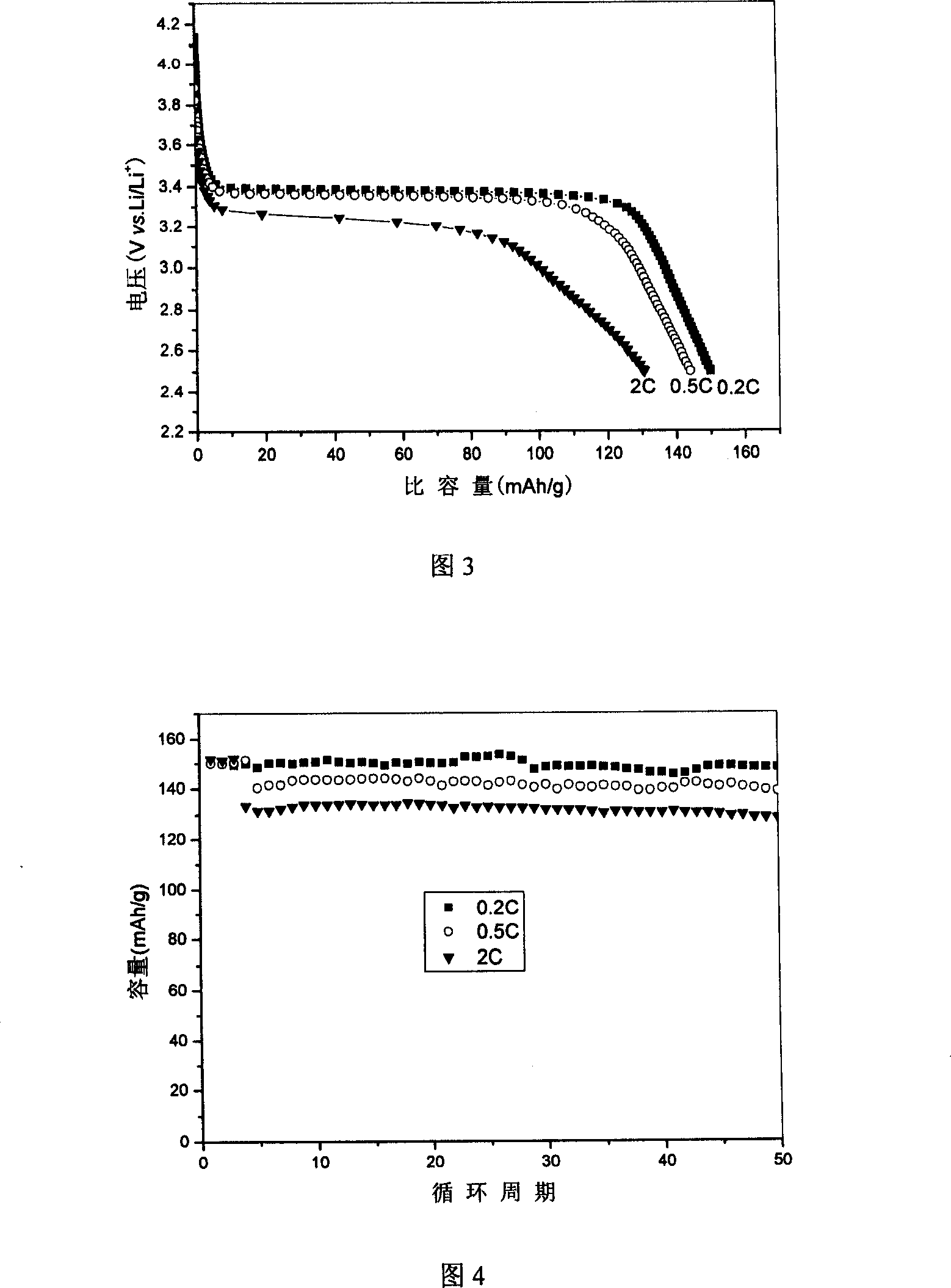
Image
Examples
Embodiment 1
[0019] 1) Weigh 0.5molFe(NO 3 ) 3 9H 2 O is dissolved in the organic solvent of ethylene glycol methyl ether, fully mixed and stirred at room temperature, is mixed with 1.5mol / L solution, takes by weighing 0.5mol lithium dihydrogen phosphate and excessive sucrose (the consumption of sucrose makes the carbon content in the product be 6%), add 50ml of distilled water to dissolve it, then mix with ethylene glycol methyl ether solution to form a uniformly dispersed sol, and stir and evaporate at 70°C for 6 hours to form a gel, and finally vacuum dry to a dry gel to obtain phosphoric acid Lithium iron precursor;
[0020] 2) Put the lithium iron phosphate precursor into a tubular resistance furnace, and pretreat it at 300°C for 5 hours under the protection of an argon gas flow with a flow rate of 5-50 liters / min;
[0021] 3) Grinding the pretreated mixture evenly, heat-treating at 725° C. for 10 hours under an argon atmosphere, and cooling down to room temperature to obtain a car...
Embodiment 2
[0026] 1) Weigh 0.5molFe(NO 3 ) 3 9H 2O is dissolved in the organic solvent of propylene glycol methyl ether, fully mixes and stirs at room temperature, is mixed with 1.5mol / L solution, takes by weighing 0.5mol potassium dihydrogen phosphate and excessive sucrose (the consumption of sucrose makes carbon content be 6% in the product) ), add 50ml of distilled water to dissolve it, then blend with propylene glycol methyl ether solution to form a uniformly dispersed sol, and stir and evaporate at 70°C for 6 hours to form a gel, and finally vacuum dry to dry gel to obtain lithium iron phosphate Precursor;
[0027] 2) Put the lithium iron phosphate precursor into a tubular resistance furnace, and pretreat it at 300°C for 5 hours under the protection of an argon gas flow with a flow rate of 5-50 liters / min;
[0028] 3) Grinding the pretreated mixture evenly, heat-treating at 725° C. for 10 hours under an argon atmosphere, and cooling down to room temperature to obtain a carbon-coa...
Embodiment 3
[0030] 1) Weigh 0.5mol ferric citrate and dissolve it in ethylene glycol methyl ether organic solvent, fully mix and stir at room temperature, and prepare a 1.5mol / L solution, weigh 0.5mol lithium dihydrogen phosphate and excess sucrose (sucrose The amount of carbon content in the product is 6%), add 50ml of distilled water to dissolve it, then mix with ethylene glycol methyl ether solution to form a uniformly dispersed sol, and stir and evaporate at 60°C for 6 hours to form a gel, and finally vacuum Dried to xerogel to obtain lithium iron phosphate precursor;
[0031] 2) Put the lithium iron phosphate precursor into a tubular resistance furnace, and pretreat it at 300°C for 5 hours under the protection of an argon gas flow with a flow rate of 5-50 liters / min;
[0032] 3) Grinding the pretreated mixture evenly, heat-treating at 725° C. for 10 hours under an argon atmosphere, and cooling down to room temperature to obtain a carbon-coated nano-lithium iron phosphate composite ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Reversible specific capacity | aaaaa | aaaaa |
| Inverse specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com