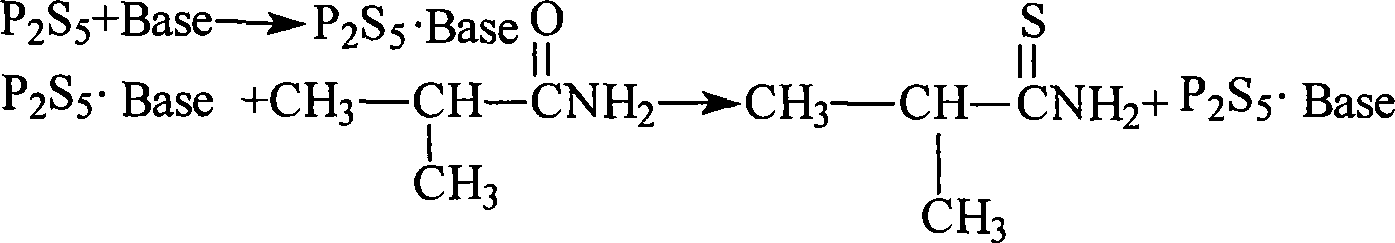Method for preparing thio-iso-butanamide
A technology of thioisobutyramide and isobutyramide, applied in the field of medicine and chemical industry, can solve the problems of low yield, difficult separation of products and raw materials, etc., and achieve the effect of stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In 600ml of ethyl acetate, add 12g of phosphorus pentasulfide, add 5.8g of anhydrous sodium carbonate in batches, and stir at 20-25°C for 30mins. After the solid was completely dissolved, 20 g of isobutyramide was added in batches, and the reactant was stirred at 25° C. for 5 hrs. The reaction mixture was filtered, the solution was concentrated, and distilled under reduced pressure. The yield was 115-118° C. / 5mmHg fraction to obtain a light yellow oily liquid, and 22.5 g of thioisobutyramide was obtained. The yield was 95.00%, and the GC content: 99.2%.
Embodiment 2
[0022] In 400ml tetrahydrofuran, add 8g phosphorus pentasulfide, add 9.35g anhydrous sodium carbonate in batches, T=23-25°C, stir for 25mim. 15.2 g of isobutyramide was added in batches. The reactant was stirred at 20-25° C. for 5 hrs. Tetrahydrofuran was recovered under reduced pressure, 50ml of water was added, the pH was adjusted to 7.5 with 5% aqueous sodium carbonate solution, extracted three times with 50mlx3 ethyl acetate, the organic layers were combined and washed with 100ml of saturated brine. Dry over anhydrous sodium sulfate, filter, recover ethyl acetate under reduced pressure, and distill the residue under reduced pressure, collect fractions at 112-118°C / 4mmHg to obtain yellow oily liquid, weight 16.2g, yield 91.2%.
Embodiment 3
[0024] Add 12.5g of phosphorus pentasulfide to 400ml of methyl tert-butyl ether, add 7.6g of anhydrous potassium carbonate in batches, and stir at 20-25°C for 30mins. 20.2 g of isobutyramide was added in batches. The reaction was stirred at 25-30°C for 5.5 hrs. The reactant was filtered, the solution was concentrated, the residue was distilled under reduced pressure, and fractions at 116-120°C / 5mmHg were collected to obtain a pale yellow oily liquid. The weight is 21.8g, the yield is 92%, and the GC content is 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com