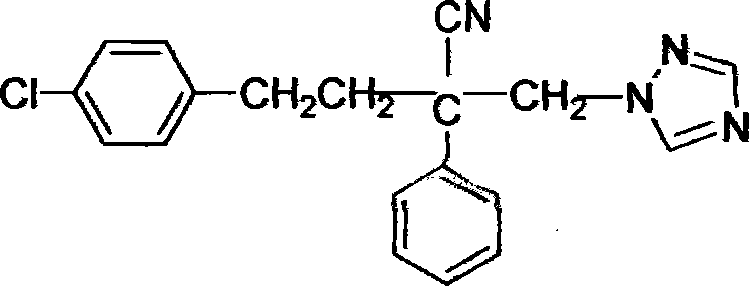
Method for preparing 2-bromomethyl-2-phenyl-4-(4-chlorophenyl)-butyronitrile
A technology of chlorophenyl and bromomethyl, which is applied in the field of preparation of 2-bromomethyl-2-phenyl-4--butyronitrile, can solve the problem of high cost, increased sewage treatment costs, and the impact on the industrial production of carbamide and other issues to achieve the effect of reducing raw material costs and saving raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 prepares 2-bromomethyl-2-phenyl-4-(4-chlorophenyl)-butyronitrile
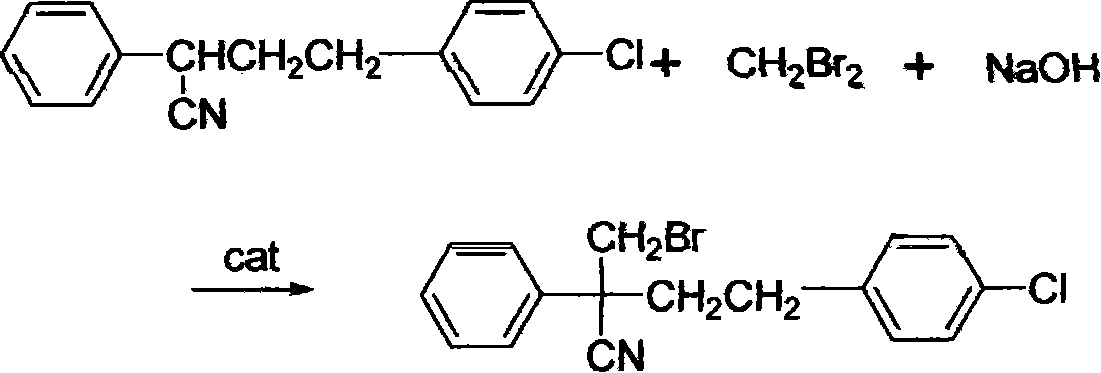
[0024] Use quaternary ammonium salt triethylbenzyl ammonium chloride as catalyst;
[0025] The feeding ratio of 2-phenyl-4-(4-chlorophenyl)butyronitrile, methylene bromide and sodium hydroxide is 0.03: 0.12: 0.108=1: 4.0: 3.6 by mole
[0026] In a 250ml three-necked reaction flask equipped with a stirrer, a condenser, a thermometer, and a dropping funnel, add 8.21g of 93.4% 2-phenyl-4-(4-chlorophenyl)-butyronitrile (equivalent to 0.03mol ), 20.86g of 100% dibromomethane (equivalent to 0.12mol), and 0.25g of triethylbenzyl ammonium chloride was added, stirring was started, the reaction temperature was controlled between 40 and 60°C, and 14.4g of 30% hydrogen was added dropwise Sodium oxide aqueous solution (equivalent to 0.108mol), dripped within 2 hours and kept at this temperature for reaction, followed by gas chromatography during the reaction until 2-phenyl-4-(4-chlorophenyl)-butyronitri...
Embodiment 2
[0027] Embodiment 2 prepares 2-bromomethyl-2-phenyl-4-(4-chlorophenyl)-butyronitrile
[0028] Use triethyl-p-chlorobenzyl ammonium chloride and tetrabutylammonium bromide as catalyst;
[0029] The feed ratio of 2-phenyl-4-(4-chlorophenyl)-butyronitrile, methylene bromide and sodium hydroxide is 0.06: 0.12: 0.216=1: 2.0: 3.6 by mole
[0030] In a 250ml three-necked reaction flask equipped with a stirrer, a condenser, a thermometer, and a dropping funnel, add 16.41g of 93.4% 2-phenyl-4-(4-chlorophenyl)-butyronitrile (equivalent to 0.06mol ), 20.86g100% dibromomethane (equivalent to 0.12mol), and add 0.6g triethyl p-chlorobenzyl ammonium chloride and 0.7g tetrabutylammonium bromide, start stirring, control the reaction temperature at 40~60°C Between, drip 17.28g50% sodium hydroxide aqueous solution (corresponding to 0.216mol), dropwise in 2 hours and insulation reaction at this temperature, track analysis with gas chromatography during the reaction, until 2-phenyl-4-( 4-Chlorop...
Embodiment 3
[0031] Example 3 Preparation of 2-bromomethyl-2-phenyl-4-(4-chlorophenyl)-butyronitrile
[0032] Use tetrabutylammonium bromide as catalyst;
[0033] The feed ratio of 2-phenyl-4-(4-chlorophenyl)-butyronitrile, methylene bromide and sodium hydroxide is 0.06: 0.15: 0.216=1: 2.5: 3.6 in moles
[0034]In a 250ml three-necked reaction flask equipped with a stirrer, a condenser, a thermometer, and a dropping funnel, add 16.41g of 93.4% 2-phenyl-4-(4-chlorophenyl)-butyronitrile (equivalent to 0.06mol ), 26.07g100% dibromomethane (equivalent to 0.15mol) and add 1.0g tetrabutylammonium bromide, start stirring, control the reaction temperature between 40~60 ℃, add dropwise 17.28g50% sodium hydroxide aqueous solution (equivalent to 0.216mol), drop it in 2 hours, and keep it warm at this temperature to react, track and analyze with gas chromatography during the reaction, until 2-phenyl-4-(4-chlorophenyl)-butyronitrile is completely converted into 2- Bromomethyl-2-phenyl-4(4-chloropheny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com