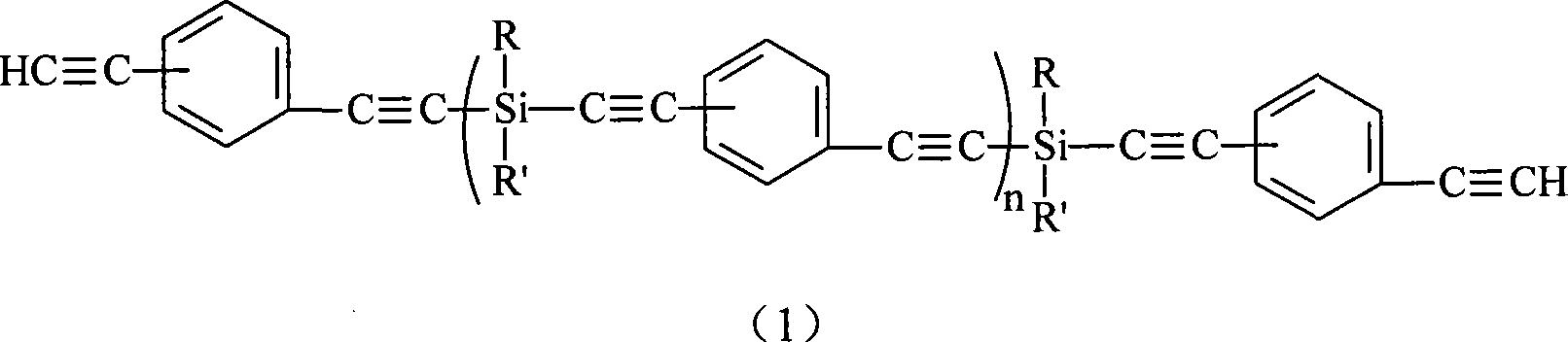
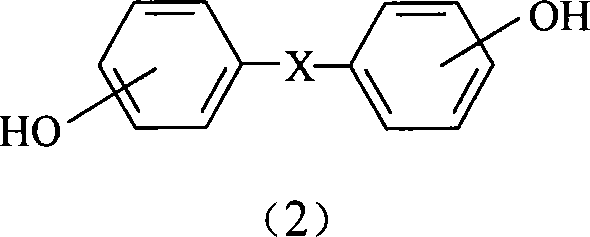
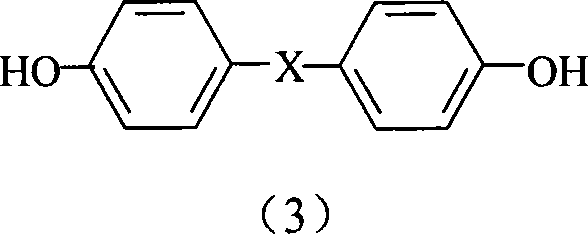
Aryne modified resin containing silicon
A technology for modifying resin and silicon aryl alkyne, applied in the field of silicon-containing aryl alkyne modified resin, can solve the problems of unsatisfactory fiber cohesion, high brittleness, poor mechanical properties of composite materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] (1) Preparation of arylethynyl benzoxazine compounds:
[0019] The bulk condensation method is used to prepare the arylethynyl benzoxazine compounds described in the present invention. This method has the advantages of simple operation, low cost, and less environmental pollution. details as follows:
[0020] Phenolic compounds, m-aminophenylacetylene and paraformaldehyde are placed in a reaction kettle, stirred, heated to 90°C to 110°C, and maintained under this condition for 20 to 40 minutes. Lower the temperature and dissolve the obtained product in halogenated hydrocarbon (such as chloroform, etc.), wash with 3 mol / L sodium hydroxide aqueous solution, and then wash with deionized water until neutral. Finally, the solvent (halogenated hydrocarbon) is evaporated to obtain the arylethynyl benzoxazine compounds of the present invention.
[0021] Wherein the recommended phenolic compound is hydroquinone, 1,4'-diphenol, 1,5-naphthalenediol or the compound shown in formul...
Embodiment 1
[0042] The synthesis of bisphenol A type arylethynyl benzoxazine [compound shown in formula (4)]:
[0043]Add stoichiometric bisphenol A 11.4g (0.0500mol), paraformaldehyde 6.01g (0.200mol), m-aminophenylacetylene 11.7g (0.100mol) ). Heat to 100°C under stirring, and react for 20 minutes. After the reaction, the product was dissolved in chloroform, washed three times with 3 mol / L sodium hydroxide solution, and then washed with deionized water until neutral. Finally, the solvent is distilled off to obtain arylethynyl benzoxazine resin. The yield was 73.7%. 1 H-NMR (CDCl 3 , TMS) δ: 1.60 (C-CH 3 ), 3.06(C≡CH), 4.56(Ar-CH 2 -N), 5.30 (O-CH 2 -N).
[0044]
[0045] Synthesis of dimethyl type silicon-containing aryne polymer [compound shown in formula (5)]:
[0046] Add treated 6.00g (0.247mol) magnesium powder and 50ml THF into a 250ml four-neck flask equipped with a stirring, constant pressure funnel and spherical condenser, and then slowly add 21.6g (0.198mol) of mag...
Embodiment 2
[0052] Synthesis of hexafluorobisphenol A type arylethynyl benzoxazine [compound shown in formula (6)]:
[0053] Add stoichiometric hexafluorobisphenol A16.8g (0.0500mol), paraformaldehyde 6.01g (0.200mol), m-aminophenylacetylene 11.7g ( 0.100mol). Heat to 100°C under stirring, and react for 20 minutes. After the reaction, the product was dissolved in chloroform, washed three times with 3 mol / L sodium hydroxide solution, and then washed with deionized water until neutral. Finally, the solvent was distilled off to obtain arylethynyl benzoxazine resin with a yield of 75.3%.
[0054] 1 H-NMR (CDCl 3 , TMS) δ: 3.06 (C≡CH), 4.60 (Ar-CH 2 -N), 5.36 (O-CH 2 -N).
[0055]
[0056] Preparation of dimethyl silicon-containing aryne modified resin DM-FBPA30:
[0057] Put 12.0g of the compound represented by the formula (6) and 28.0g of the compound represented by the formula (5) into a reaction kettle equipped with a stirrer, a thermometer and a reflux condenser, slowly raise t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com