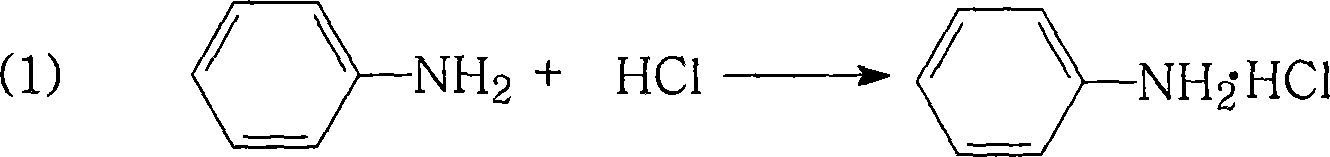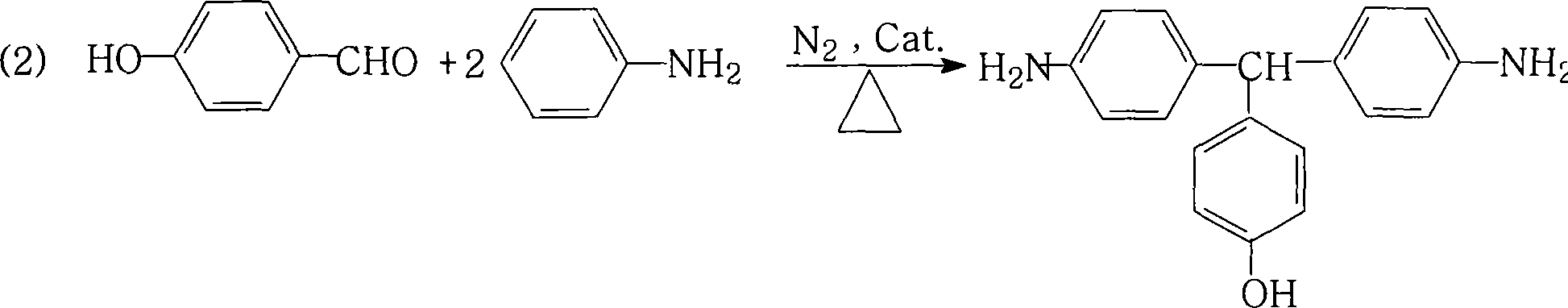Method for preparing 4,4'-diamino-4'-hydroxytriphenylmethane
A technology of hydroxytriphenylmethane and p-hydroxybenzaldehyde, which is applied in 4 fields, can solve the problems of low yield, decreased yield, and many operation steps, and achieve the effect of less equipment investment, low cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 18.6 grams (0.1 moles) of newly distilled aniline into the reaction kettle, stir at room temperature, add 19.8 grams (0.2 moles) of 37% hydrochloric acid aqueous solution dropwise, react at room temperature for 0.1 hour, add 10 milliliters of toluene, stir, and heat up Reflux to separate the water until no more water comes out, cool naturally, and precipitate a white solid product, filter, and dry in vacuum to obtain 20.7 g of white aniline hydrochloride crystals with a melting point of 196.3°C to 198.0°C and a purity of 99.1%. The obtained crystal weight of aniline hydrochloride and the theoretical yield (25.9 g) calculated that the yield of aniline hydrochloride was 80%.
Embodiment 2
[0046] Add 18.6 grams (0.1 moles) of newly distilled aniline to the reactor, stir at room temperature, add dropwise 29.8 grams (0.3 moles) of 37% hydrochloric acid aqueous solution, and react at room temperature for 4 hours, add 400 milliliters of xylene, stir, and heat Heat up and reflux to separate water until no more water comes out, cool naturally, and a white solid product is precipitated, filtered, and vacuum-dried to obtain 23.3 g of white aniline hydrochloride crystals with a melting point of 197.5°C to 198.3°C and a purity of 99.8%. According to According to the actual obtained crystal weight of aniline hydrochloride and the theoretical yield (25.9 grams), the yield of aniline hydrochloride was calculated to be 90%.
Embodiment 3
[0048]Add 18.6 grams (0.1 mol) of newly distilled aniline to the reactor, stir at room temperature, add dropwise 24.6 grams (0.25 mol) of 37% aqueous hydrochloric acid solution, and react at room temperature for 1 hour, add 100 ml of dichlorobenzene, and stir , heat up and reflux to separate water until no more water comes out, cool naturally, and a white solid product is precipitated, filtered, and vacuum-dried to obtain 24.4 g of white aniline hydrochloride crystals with a melting point of 197.2°C to 198.1°C and a purity of 99.8% According to the crystal weight of aniline hydrochloride actually obtained and the theoretical yield (25.9 grams), the yield of aniline hydrochloride was calculated to be 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com