Method for preparing hyper branched supermolecule main block based on modification cyclodextrin
A technology of cyclodextrin and supramolecule, which is applied in the field of preparation of hyperbranched supramolecular body, achieves the effects of low viscosity, novel structure and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
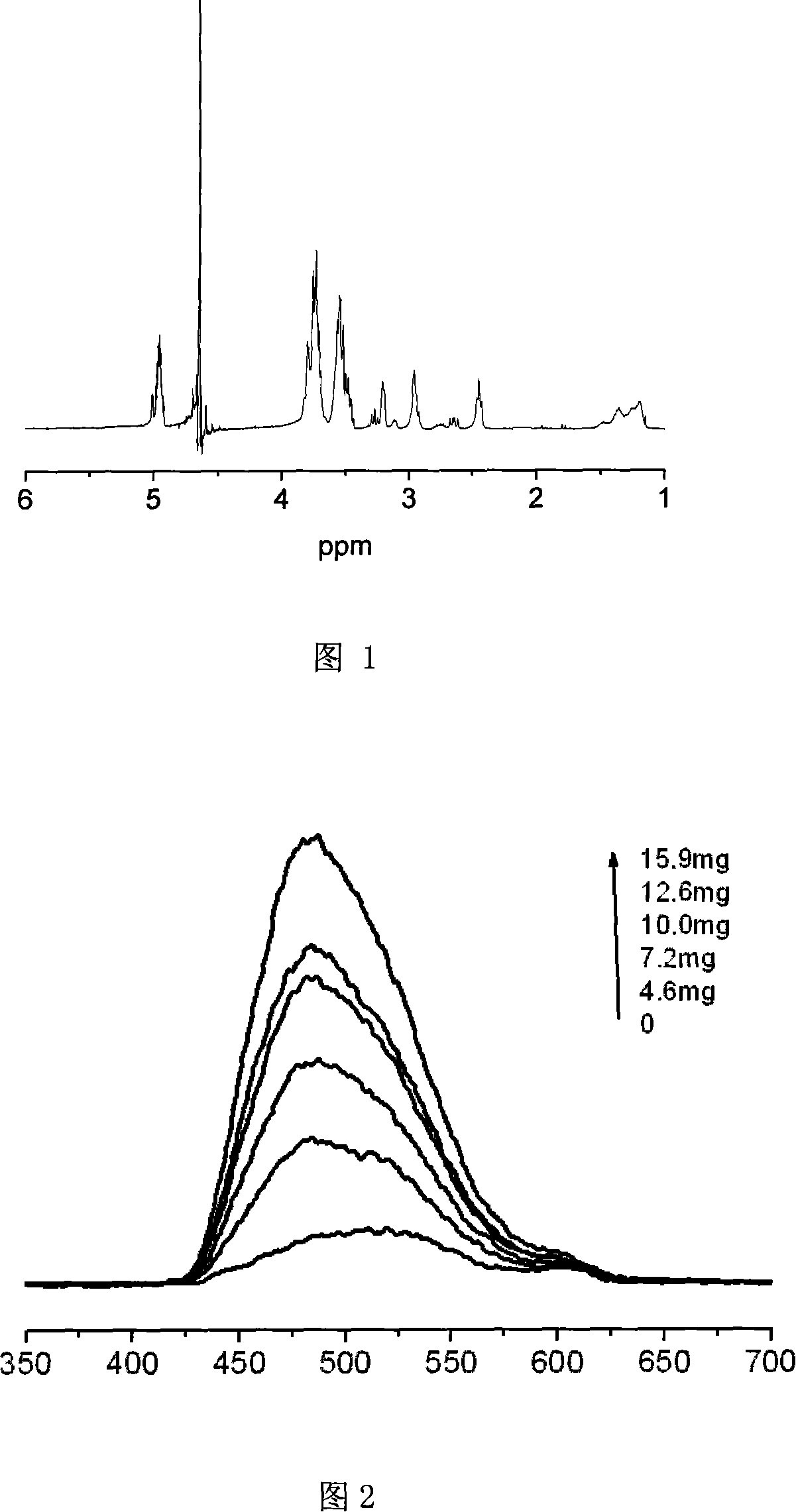
Image
Examples
Embodiment 1
[0020] Step a: Dissolve 30 g of cyclodextrin in 720 ml of aqueous solution, then add 7.8 g of p-toluenesulfonyl chloride, stir at room temperature for 3-5 hours, and the reaction is solid-liquid two-phase. After 3 hours, 120 milliliters of 2.5 mol / liter sodium hydroxide solution was added, the solution became clear, and a little white insoluble matter was removed by filtration. About 9 grams of ammonium chloride was added to adjust the pH to about 8, and white insoluble matter was precipitated in the solution. Place in the refrigerator overnight, filter with suction to obtain a white solid, which is repeatedly recrystallized 3 times. The final product was dried in a vacuum oven at 60 degrees Celsius for 24 hours, and the yield was 16.7%.
[0021] Step b: In N,N-dimethylformamide, 5 g of the prepared p-toluenesulfonylated β-cyclodextrin was dissolved in 20 ml of N,N-dimethylformamide, Add 10 g of hexamethylenediamine and stir for 4 hours at 70°C. Precipitation with 200 mL of...
Embodiment 2
[0024] Step a: Dissolve 30 g of cyclodextrin in 720 ml of aqueous solution, then add 7.8 g of p-toluenesulfonyl chloride, stir at room temperature for 3-5 hours, and the reaction is solid-liquid two-phase. After 3 hours, 120 milliliters of 2.5 mol / liter sodium hydroxide solution was added, the solution became clear, and a little white insoluble matter was removed by filtration. About 9 grams of ammonium chloride was added to adjust the pH to about 8, and white insoluble matter was precipitated in the solution. Place in the refrigerator overnight, filter with suction to obtain a white solid, which is repeatedly recrystallized 3 times. The final obtained product was dried in a vacuum oven at 60 degrees Celsius for 24 hours, and the yield was 16.7%.
[0025] Step b: In N,N-dimethylformamide, 5 g of the prepared p-toluenesulfonylated β-cyclodextrin was dissolved in 20 ml of N,N-dimethylformamide, Add 10 g of hexamethylenediamine and stir for 4 hours at 70°C. Precipitate with 20...
Embodiment 3
[0028] Step a: Dissolve 30 g of cyclodextrin in 720 ml of aqueous solution, then add 7.8 g of p-toluenesulfonyl chloride, stir at room temperature for 3-5 hours, and the reaction is solid-liquid two-phase. After 3 hours, 120 milliliters of 2.5 mol / liter sodium hydroxide solution was added, the solution became clear, and a little white insoluble matter was removed by filtration. About 9 grams of ammonium chloride was added to adjust the pH to about 8, and white insoluble matter was precipitated in the solution. Place in the refrigerator overnight, filter with suction to obtain a white solid, which is repeatedly recrystallized 3 times. The final obtained product was dried in a vacuum oven at 60 degrees Celsius for 24 hours, and the yield was 16.7%.
[0029]Step b: In N,N-dimethylformamide, 5 g of the prepared p-toluenesulfonylated β-cyclodextrin was dissolved in 20 ml of N,N-dimethylformamide, Add 10 g of hexamethylenediamine and stir for 4 hours at 70°C. Precipitate with 200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com