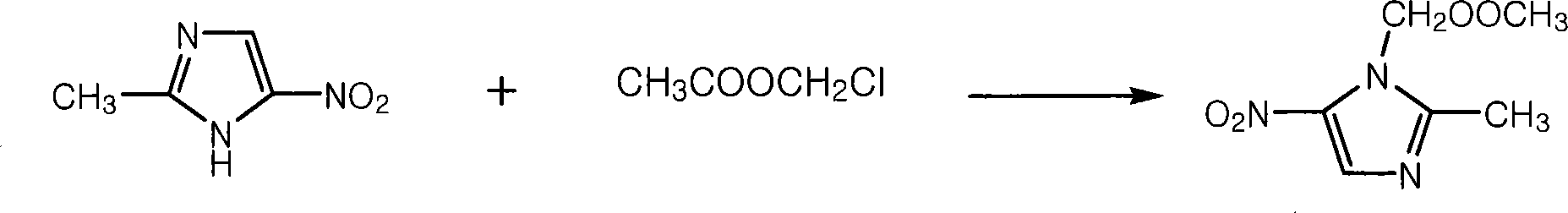Preparation and purification method for optical enantiomer of ornidaxole
An ornidazole optical and enantiomeric technology is applied in the field of preparation and purification of ornidazole optical enantiomer, and can solve the problem of many side reactions of chiral ethylene oxide, low yield, and optical enantiomer of ornidazole. problems such as large body cost, to achieve the effects of high yield, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] a. Preparation of nitroimidazole methyl acetate
[0019] Mix 30kg of 2-methyl-5-nitroimidazole, 41kg of anhydrous sodium carbonate, 200L of ethyl acetate and a small amount of phase transfer catalyst in a 500L enamel reaction kettle, slowly add 40kg of chloromethyl acetate, and heat to Reflux for 7 hours, cool to room temperature, filter, wash with water, add anhydrous sodium sulfate to dry, recover ethyl acetate to dryness, and obtain 56 kg of nitroimidazolium acetate.
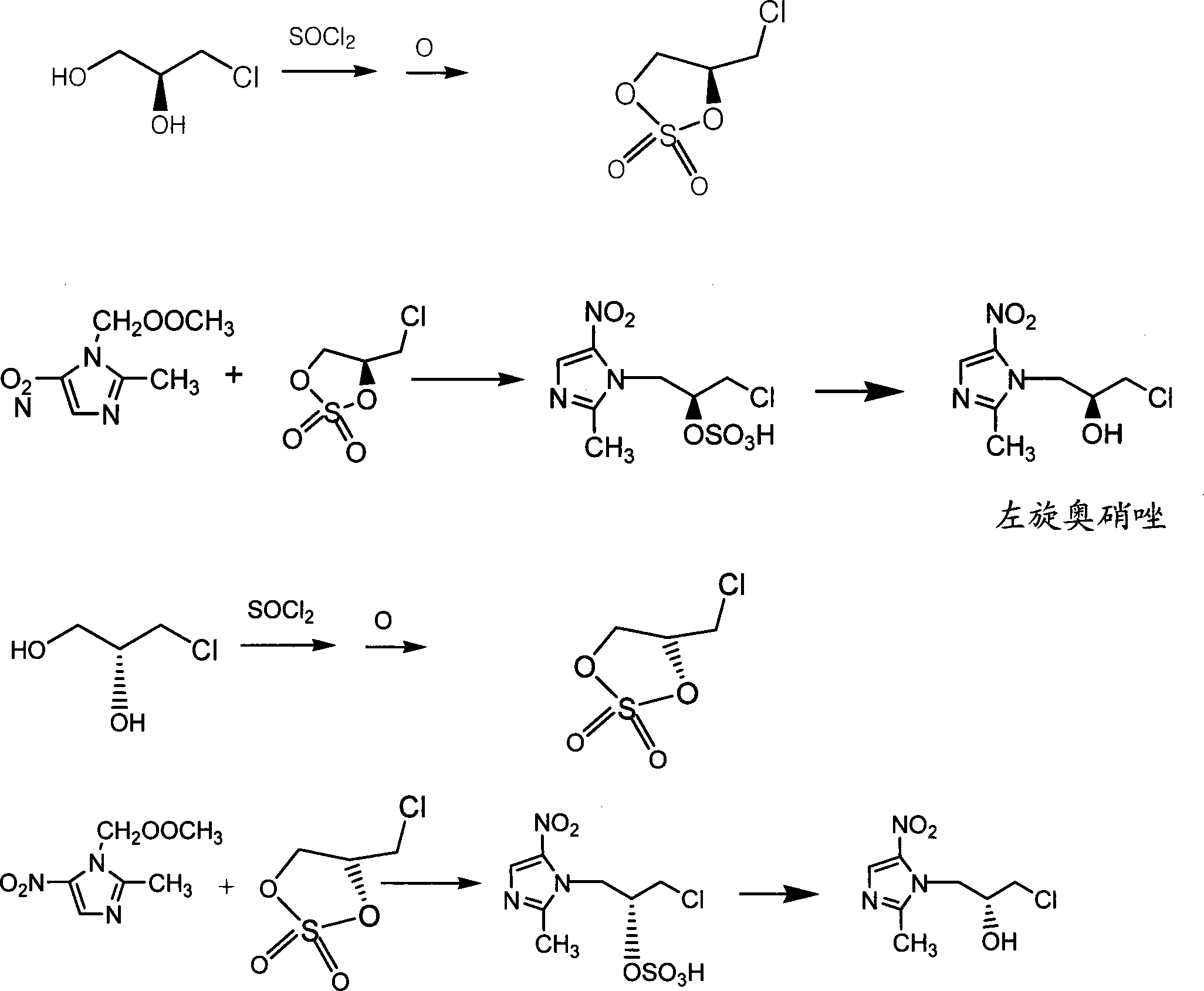
[0020] b. Preparation of Cyclic Sulfate
[0021] Dissolve 110kg of S-3-chloro-1,2-propanediol in 500L of methylene chloride in a 1000L enamel reaction kettle, add dropwise a mixed solution of 200kg of thionyl chloride and 100L of methylene chloride under cooling with ice-salt water, and after the addition is completed, liter High temperature to reflux, react for 1.5 hours. Cool to -10°C-0°C, then slowly add 100L of 30% hydrogen peroxide, keep warm at 0-10°C for 3 hours, separate liquid, wash with wat...
Embodiment 2
[0025] c. Preparation of nitroimidazole methyl acetate
[0026] Mix 25kg of 2-methyl-5-nitroimidazole, 37kg of anhydrous sodium carbonate, 150L of acetonitrile and a small amount of phase transfer catalyst in a 300L enamel reaction kettle, slowly add 40kg of chloromethyl acetate, and heat to reflux for 6 hours, filter, recover acetonitrile, dissolve the residue with ethyl acetate, wash with water, add anhydrous sodium sulfate to dry, recover ethyl acetate to dryness, and obtain 46.1 kg of nitroimidazolium acetate.
[0027] d. Preparation of Cyclic Sulfate
[0028] Dissolve 110kg of R--3-chloro-1,2-propanediol in 500L of dichloromethane in a 1000L enamel reaction kettle, and add dropwise a mixed solution of 200kg of thionyl chloride and 100L of dichloromethane under cooling with ice-salt water. After the addition is complete, Raise the temperature to reflux and react for 1.5 hours. Cool to -10°C-0°C, then slowly add 100L of 30% hydrogen peroxide, keep warm at 0-10°C for 3 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com