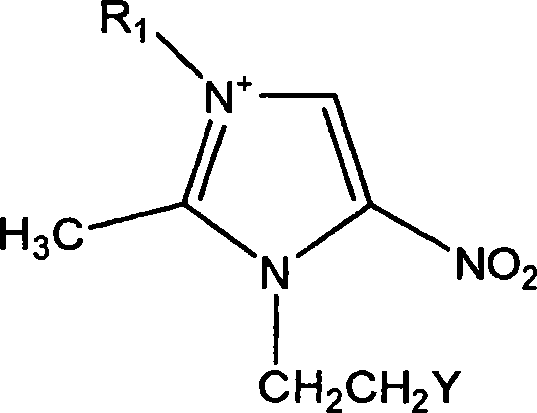
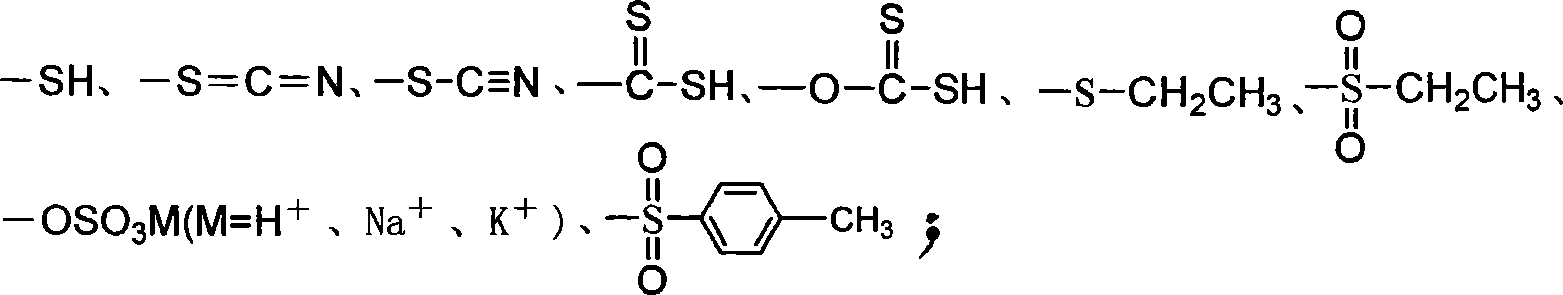
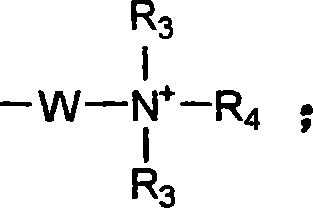Heterocyclic organic sulfide germicide and its preparation method
A fungicide, organic sulfur technology, applied in the directions of fungicides, biocides, biocides, etc., can solve the problems of unsatisfactory effect, limited application, inability to kill SRB, etc., and achieve better bactericidal effect and good bactericidal performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 51.8g (0.3mol) of metronidazole and 50ml of concentrated sulfuric acid and add them to the reaction flask. After stirring evenly, slowly add an aqueous solution of ammonium bromide (38g (0.39mol) dissolved in 30g of water) dropwise. The temperature was raised to control the reaction temperature to be about 120° C., and the reaction was performed for 12 hours. Then cool to room temperature, adjust the pH value to about 4 with 40% NaOH solution, produce a large amount of light yellow solid precipitation, filter with suction, wash with water, and dry to obtain the dry product 1-(2-bromoethyl)-2-methyl - 62.1 g of 5-nitroimidazole (A), yield 87.6%. mp: 79-80°C (literature value mp: 80-81°C).
[0027] Weigh 46.8g (0.2mol) of A, 17.9g (0.22mol) of sodium thiocyanate, and 200ml of ethyl acetate, and heat to reflux for 6h. Suction filtration, washing several times with ethyl acetate, and then washing several times with water, after drying, 32.8g of white solid 1-(2-thio...
Embodiment 2
[0030] Weigh 42.4g (0.2mol) of B (see Example 1 for its synthesis), 43g (0.2mol) of dodecyldimethyl tertiary amine, and 50g of ethanol, stir evenly and heat up to about 40°C, and dropwise add epoxy Chloropropane 18.7g (0.2mol), after adding, reflux reaction for 6h, then add an appropriate amount of water to prepare a solution with a certain effective content, which is the heterocyclic organosulfur fungicide (HOS-2).
Embodiment 3
[0032] Weigh 51.8g (0.3mol) of metronidazole, stir, add 70g (0.3mol) of chlorosulfonic acid dropwise at room temperature, control the temperature < 40°C, react at about 40°C for 2h after the addition, and remove excess chlorosulfonic acid under reduced pressure , to obtain sulfonated products.
[0033] Take by weighing 50.5g (0.2mol) of the above-mentioned sulfonated product, 43g (0.2mol) of dodecyl dimethyl tertiary amine, and 50g of ethanol, stir well and heat up to about 40°C, and dropwise add epichlorohydrin 18.7g ( 0.2mol), after adding, reflux for 6 hours, then add appropriate amount of water to prepare a solution with a certain effective content, which is the heterocyclic organosulfur fungicide (HOS-3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com