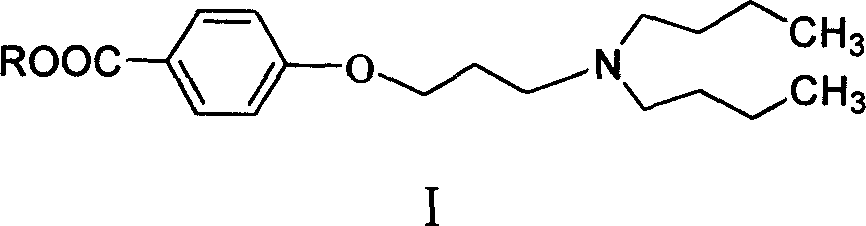
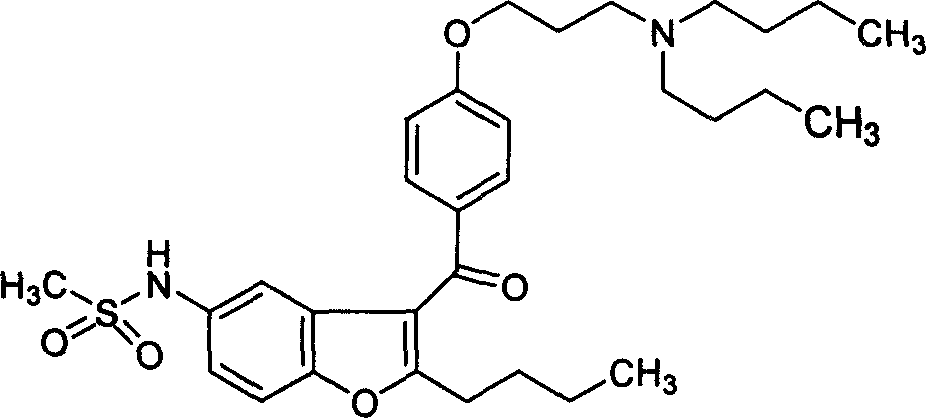
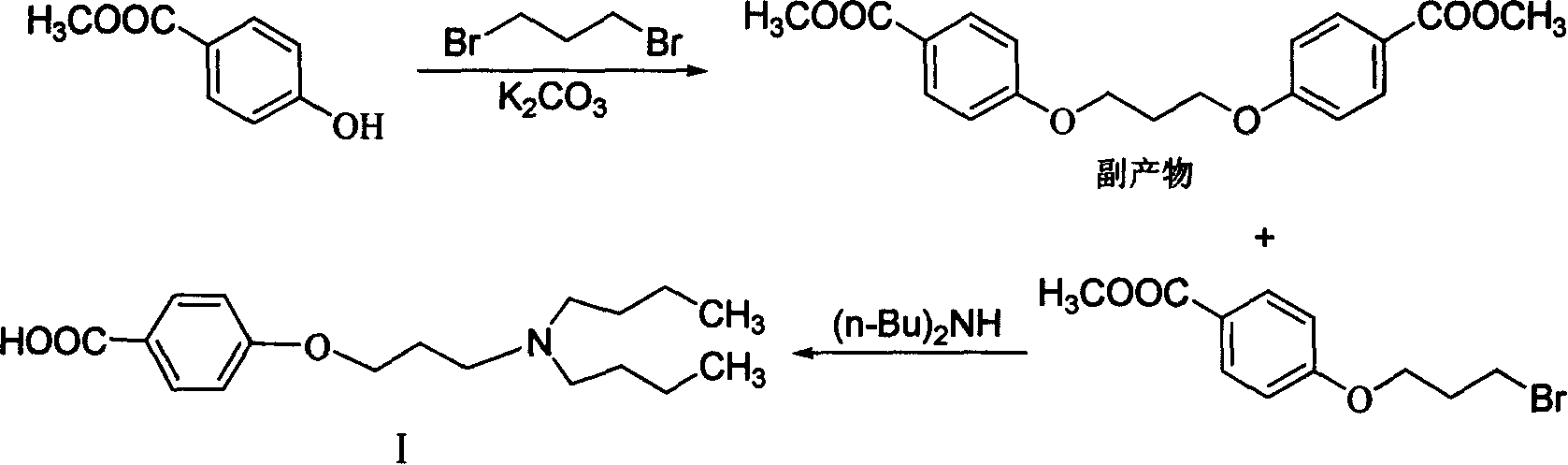
Novel method of producing dronedarone key intermediate
A compound and basic technology, which is applied in the field of preparation of 4-[3-propoxy]-benzoic acid alkyl esters, can solve problems such as no effective solution, and achieves prevention of by-product generation, high yield, and high efficiency. Strong control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under nitrogen protection, 172 g of 1-chloro-3-bromopropane was added to 300 ml of butanone, and 158 g of anhydrous potassium carbonate was added. The temperature was raised to reflux, and a mixed solution of 200 milliliters of butanone and 152 grams of methyl p-hydroxybenzoate was added dropwise. After the dropwise addition was completed, the reaction was refluxed for 5 hours. Cool to room temperature, filter with suction, and wash the filter cake with a small amount of butanone. The filtrates were combined, and the solvent was evaporated under reduced pressure to obtain methyl 4-(3-chloropropoxy)benzoate as 205 g of off-white solid, with a yield of 90%. HPLC purity 98.5%.
Embodiment 2
[0036] Under nitrogen protection, 172 g of 1-chloro-3-bromopropane was added to 300 ml of butanone, and 158 g of anhydrous potassium carbonate was added. The temperature was raised to reflux, and a mixed solution of 200 milliliters of butanone and 152 grams of methyl p-hydroxybenzoate was added dropwise. After the dropwise addition was completed, the mixture was cooled to room temperature and stirred for 24 hours. Suction filtration, the filter cake was washed with a small amount of butanone. The filtrates were combined, and the solvent was distilled off under reduced pressure to obtain methyl 4-(3-chloropropoxy)benzoate as 180 g of off-white solid, with a yield of 79%. HPLC purity 98.0%.
Embodiment 3
[0038] Under nitrogen protection, 172 g of 1-chloro-3-bromopropane was added to 250 ml of toluene, and 50 g of sodium hydroxide was added. The temperature was raised to reflux, and a mixed solution of 150 milliliters of toluene and 152 grams of methyl p-hydroxybenzoate was added dropwise. After the dropwise addition was completed, the reaction was refluxed for 4 hours. Cool to room temperature, filter with suction, and wash the filter cake with a small amount of toluene. The filtrates were combined, and the toluene was evaporated under reduced pressure to obtain methyl 4-(3-chloropropoxy)benzoate as 172 g of off-white solid, with a yield of 75.5%. HPLC purity 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com