Method for producing ultraviolet light excitated single phase white radiation fluorescent powder
A fluorescent powder and ultraviolet light technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of high reaction energy consumption, easy generation of heterogeneous phases, uneven mixing of raw materials, etc., to achieve good luminescence performance, easy to obtain raw materials, Operational Security Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
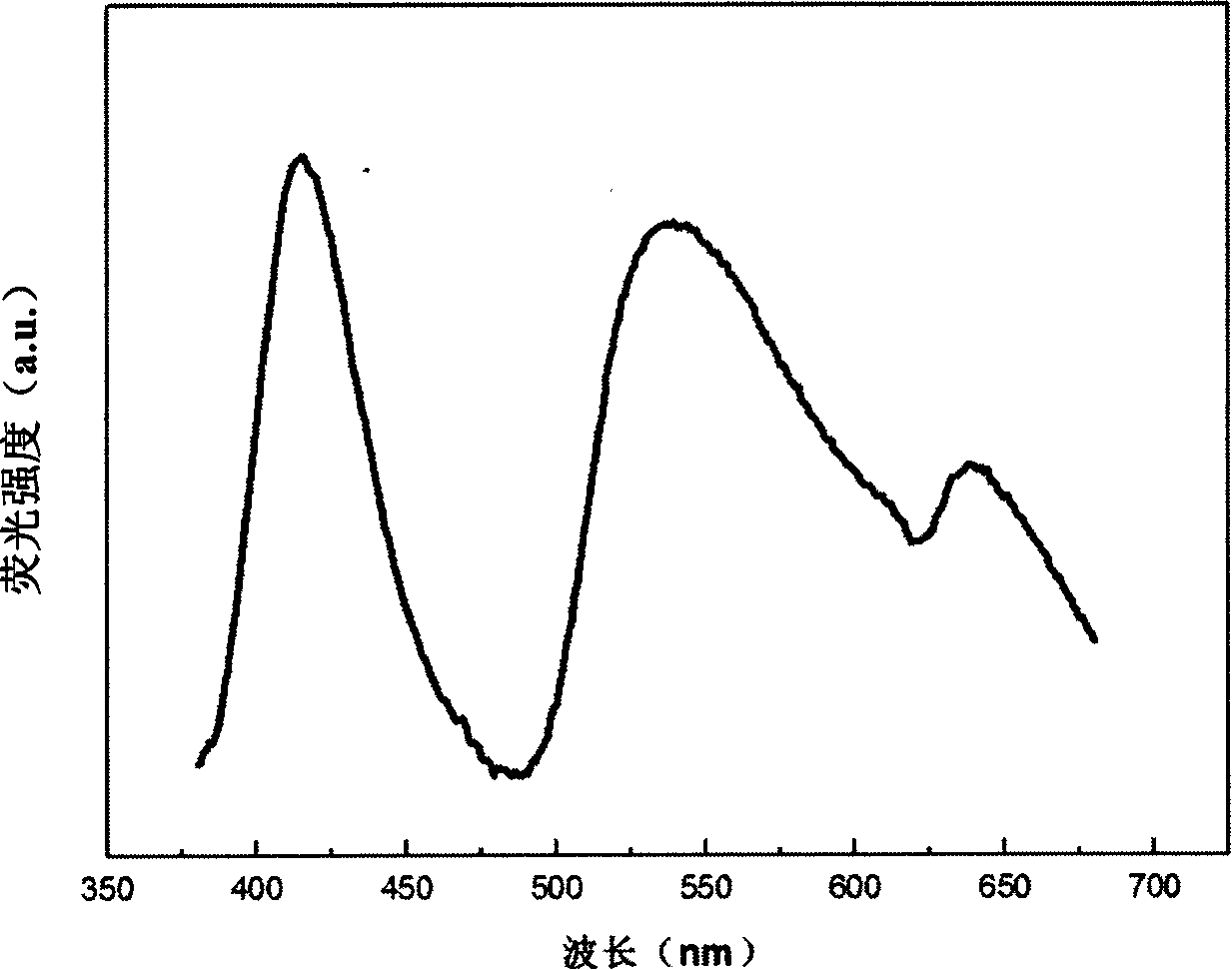
[0029] Example 1Sr 0.995 Eu 0.005 Zn 1.995 mn 0.005 (PO 4 ) 2
[0030] Weigh SrCO 3 (analytical pure) 2.938g, ZnO (analytical pure) 3.247g, MnCO 3 (99.99%) 0.0115g and Eu 2 o 3 (99.99%) 0.0176 g. The above raw materials were dissolved in excess concentrated nitric acid. After fully mixing the solution, add 0.55 g of salicylic acid complexing agent. After stirring evenly, add urea 0.24g, PEG0.3g, NH 4 h 2 PO 4 (Analytical pure) 4.601g, and fully stirred evenly. The solution was heated at 60°C until all the water in the solution was evaporated to form a gel. In the process of heating the solution, add ammonia water to the solution to control the pH of the solution to be about 4. The obtained gel was burned in an air atmosphere at 900° C. for 5 hours to obtain a precursor powder. The obtained precursor powder was sintered in a reducing atmosphere at 1000° C. for 2 hours to obtain the desired phosphor.
[0031] figure 1 It is the excitation spectrum of the fluor...
Embodiment 2
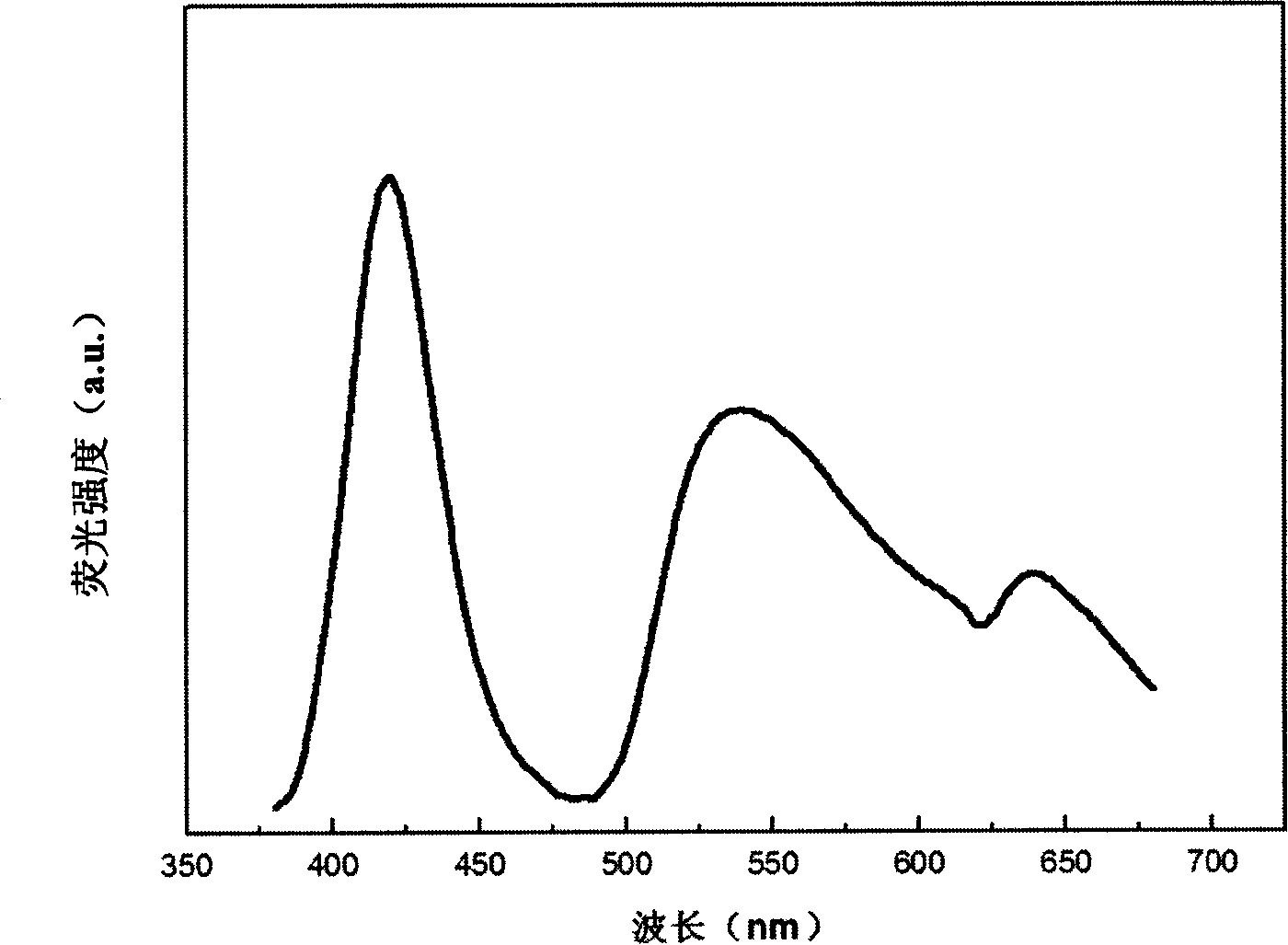
[0032] Example 2Sr 0.99 Eu 0.01 Zn 1.99 mn 0.01 (PO 4 ) 2
[0033] Weigh SrCO 3 (analytical pure) 2.923g, ZnO (analytical pure) 3.239g, MnCO 3 (99.99%) 0.023g and Eu 2 o 3 (99.99%) 0.0352 g. The above raw materials were dissolved in excess concentrated nitric acid. After fully mixing the solution, add 0.7 g of salicylic acid complexing agent. After stirring evenly, add urea 0.6g, PEG0.3g, NH 4 h 2 PO 4(Analytical pure) 4.601g, and fully stirred evenly. The solution was heated at 70°C until all the water in the solution was evaporated to form a gel. In the process of heating the solution, add ammonia water to the solution to control the pH value of the solution to be about 7. The obtained gel was burned in an air atmosphere at 900° C. for 2 hours to obtain a precursor powder. The obtained precursor powder was sintered in a reducing atmosphere at 1000° C. for 15 hours to obtain the desired phosphor.
[0034] figure 2 It is the excitation spectrum of the fluor...
Embodiment 3
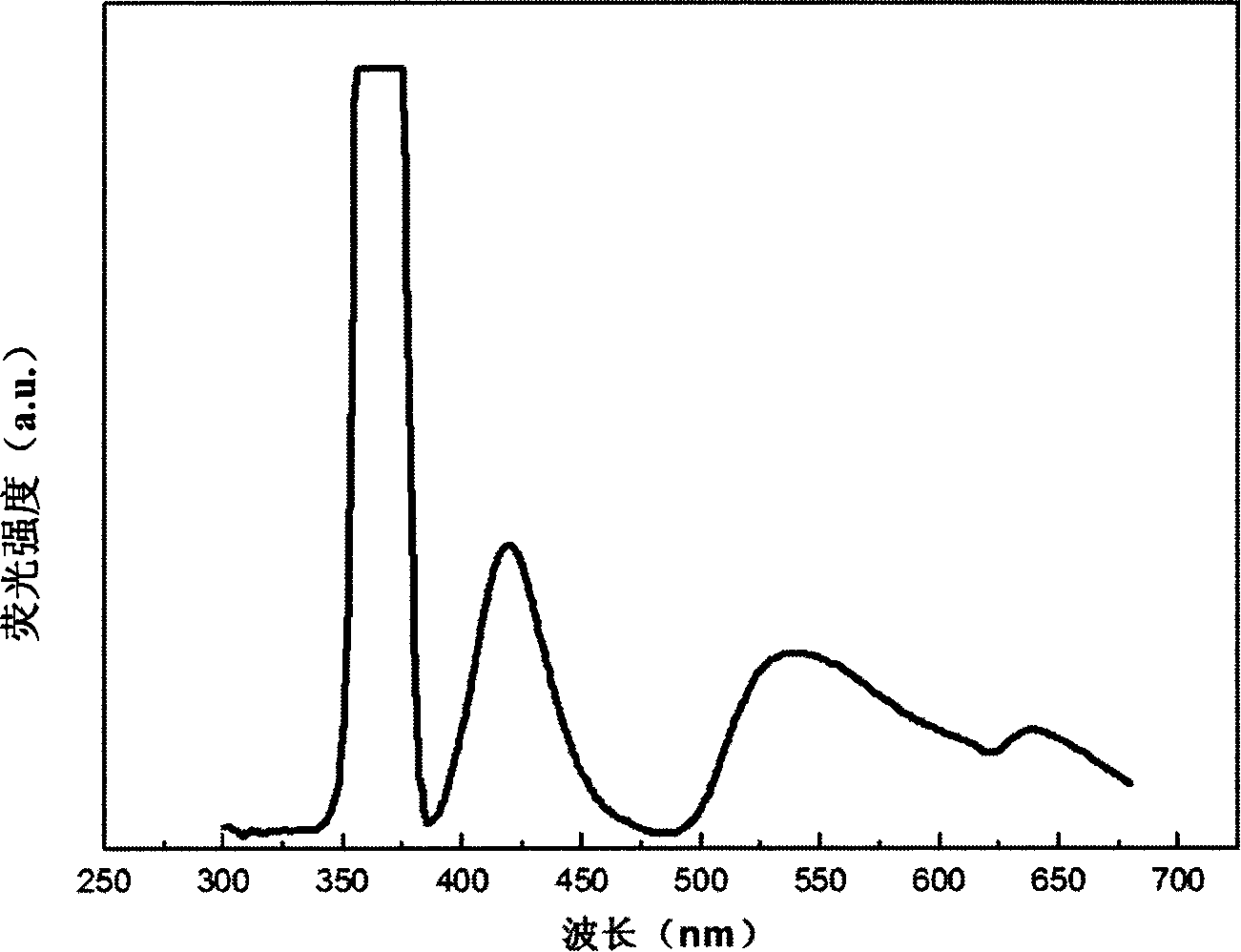
[0035] Example 3Sr 0.99 Eu 0.01 Zn 1.99 mn 0.01 (PO 4 ) 2
[0036] Weigh SrCO 3 (analytical pure) 2.923g, ZnO (analytical pure) 3.223g, MnCO 3 (99.99%) 0.046g and Eu 2 o 3 (99.99%) 0.0352 g. The above raw materials were dissolved in excess concentrated nitric acid. After fully mixing the solution, add 2 g of salicylic acid complexing agent. After stirring evenly, add urea 1g, PEG 1g, NH 4 h 2 PO 4 (Analytical pure) 4.601g, and fully stirred evenly. The solution was heated at 90°C until all the water in the solution was evaporated to form a gel. In the process of heating the solution, add ammonia water to the solution to control the pH value of the solution to be about 5. The obtained gel was burned in an air atmosphere at 900° C. for 3 hours to obtain a precursor powder. The obtained precursor powder was sintered in a reducing atmosphere at 1000° C. for 25 hours to obtain the desired phosphor.
[0037] Figure 4 It is the excitation spectrum of the fluoresce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com