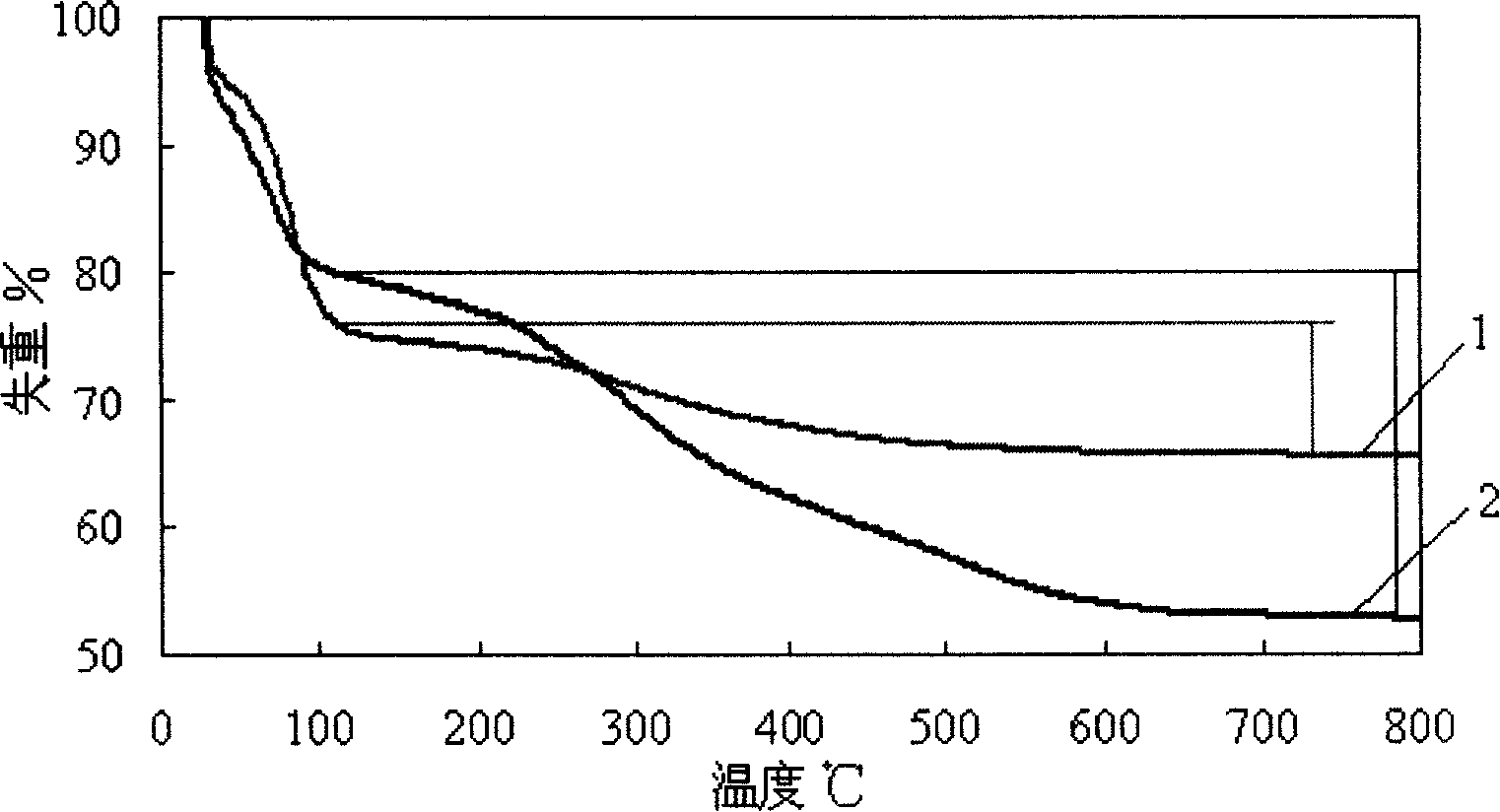
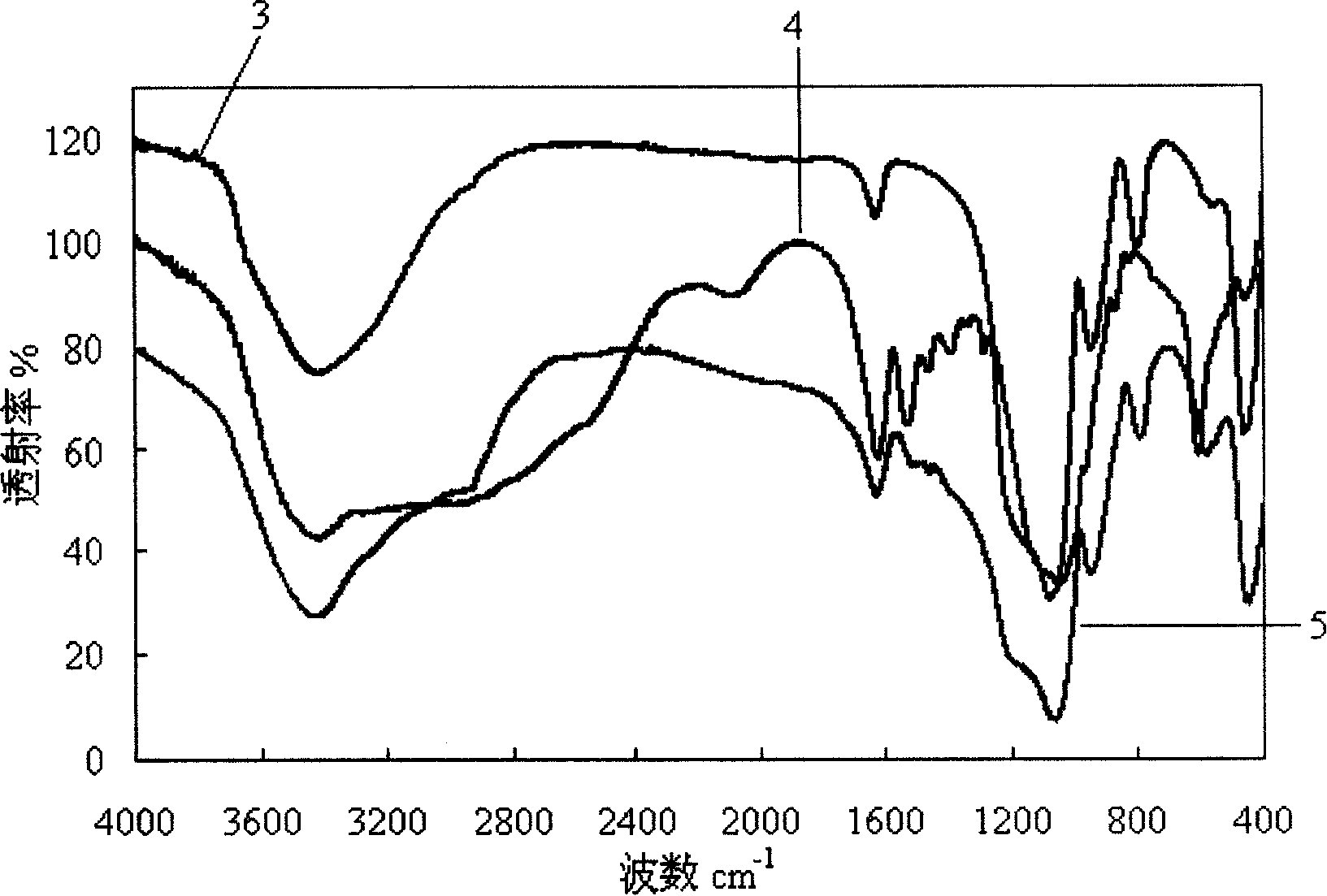
Medicine-loading sustained-release micro-capsule and preparing method thereof
A slow-release microcapsule and drug-carrying technology, which is applied in the direction of pharmaceutical formulations, drug delivery, and medical preparations of non-active ingredients, can solve the problems of short effective action time, unsatisfactory needs, and short sustained-release period, etc., and achieve good results. Biocompatibility, good physical and chemical properties, smooth drug release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: 0.2g drug gentamicin sulfate is dissolved in 2ml mass fraction and is 6% hydrochloric acid, and adds 0.04g water-soluble surfactant polyoxyethylene sorbitan monooleate, mixes uniformly to form drug- Aqueous solution A of surfactant-precipitating agent, wherein the drug concentration is about 100mg / ml, and the concentration of water-soluble surfactant is about 0.02g / ml; add 2.0g of oil-soluble surfactant sorbitan monooleate to 50ml In the water-insoluble non-polar oil solvent cyclohexane, mix uniformly to form a surfactant-oil solvent solution B, wherein the concentration of the oil-soluble surfactant is about 0.04g / ml; under stirring conditions, the solution A Slowly add in solution B, the volume ratio of solution A and solution B is 1:25, keep stirring until a stable water / oil type emulsion C is formed; 25% of the mass), and continued to stir for 24 hours, so that the silicon source material was fully hydrolyzed and polycondensed, and a silica coating lay...
Embodiment 2
[0036] Embodiment 2: Add 0.4g drug gentamycin sulfate and 0.04g water-soluble surfactant polyoxyethylene sorbitan monooleate to 2ml mass fraction of 6% hydrochloric acid, mix uniformly to form drug-surfactant Aqueous solution A of agent-precipitating agent, wherein drug concentration is about 200mg / ml, and water-soluble surfactant concentration is about 0.02g / ml; In 50ml of water-insoluble non-polar oil solvent cyclohexane, mix uniformly to form surfactant-oil solvent solution B, wherein the concentration of oil-soluble surfactant is about 0.04g / ml; under stirring conditions, the solution A is slowly added to solution B, the volume ratio of solution A to solution B is 1:25, and the stirring is continued until a stable water / oil emulsion C is formed; 2ml of tetraethyl orthosilicate, a silicon source material, is added to emulsion C (according to aqueous solution 25% of the mass of A), and continued to stir for 30 hours, so that the silicon source material was fully hydrolyzed a...
Embodiment 3
[0037] Embodiment 3: 0.2g drug salbutamol sulfate is dissolved in 2ml mass fraction and be in the hydrochloric acid of 6%, and add 0.04g water-soluble surfactant polyoxyethylene sorbitan monooleate, mix uniformly to form drug-surfactant Aqueous solution A of agent-precipitating agent, wherein drug concentration is about 100mg / ml, water-soluble surfactant concentration is about 0.02g / ml; 2.0g oil-soluble surfactant sorbitan monooleate is added into 50ml insoluble In the non-polar oil solvent cyclohexane of water, mix evenly to form a surfactant-oil solvent solution B, in which the concentration of oil-soluble surfactant is about 0.04g / ml; under stirring conditions, slowly add solution A In solution B, the volume ratio of solution A to solution B is 1:25, and the stirring is continued until a stable water / oil emulsion C is formed; 25% meter), and continue to stir for 24 hours, so that the silicon source material is fully hydrolyzed and polycondensed, and a silica coating layer i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com