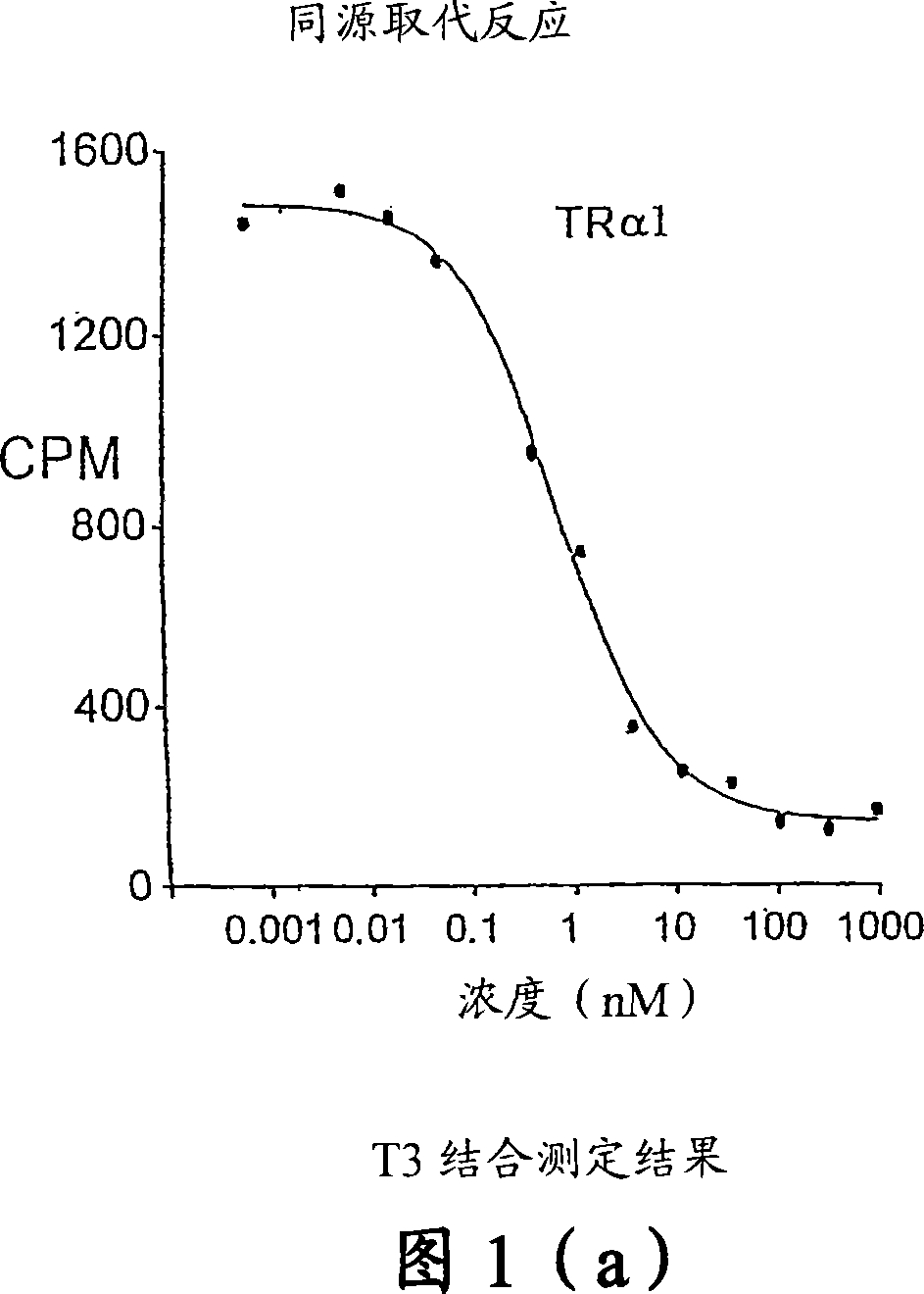
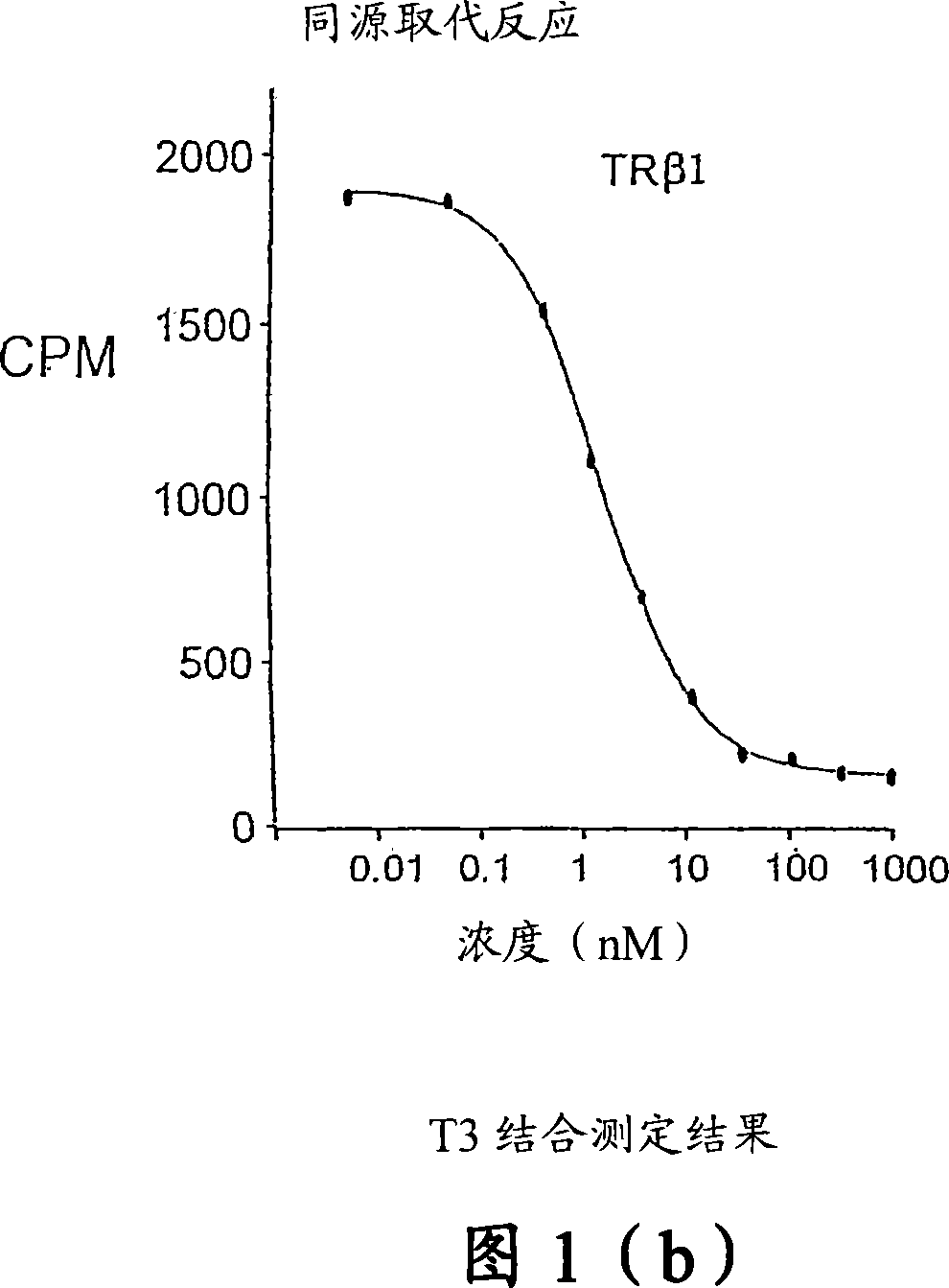
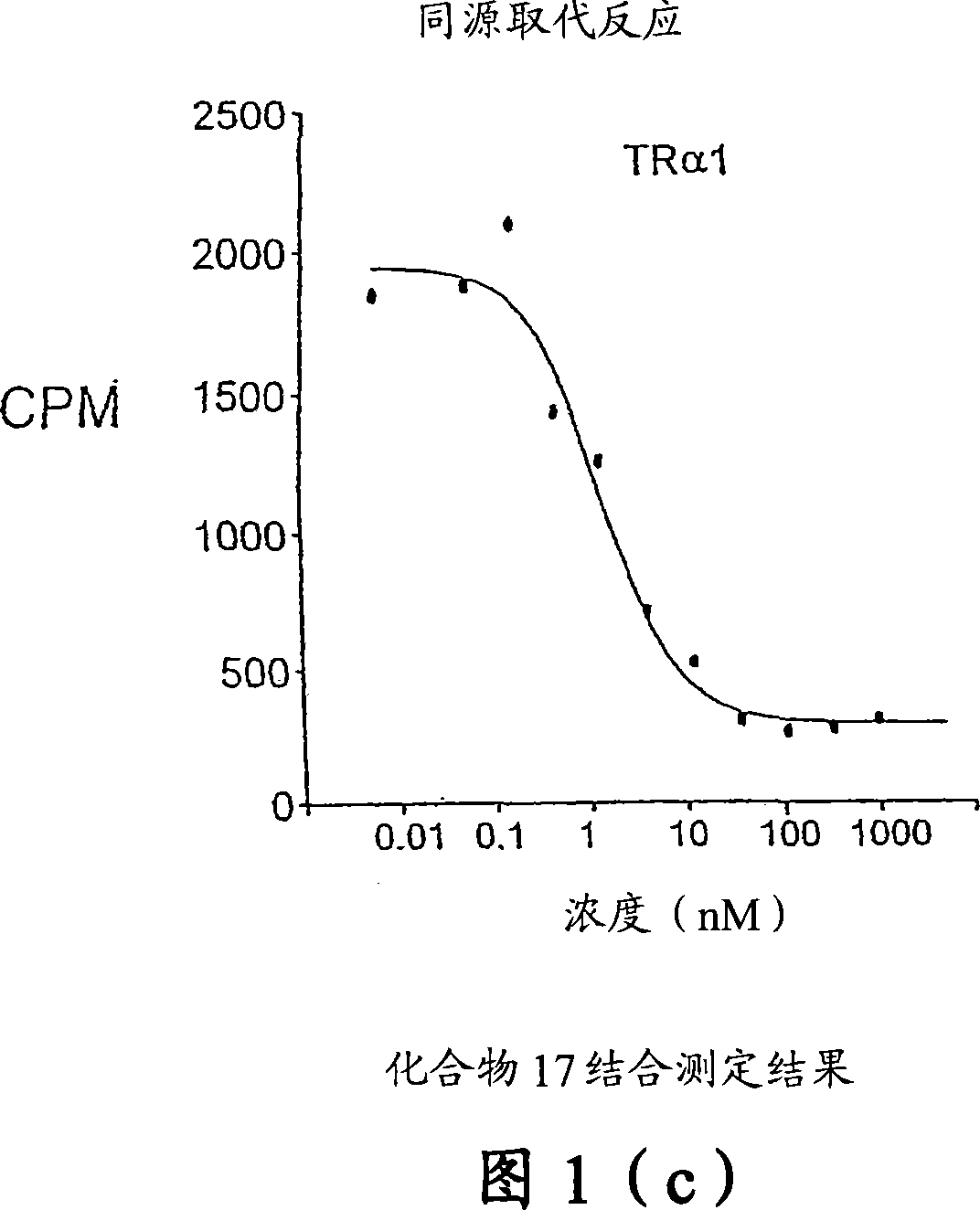
Novel phosphinic acid-containing thyromimetics
A halogen and trifluoromethyl technology, which is applied in the field of new phosphinic acid-containing thyromimetic drugs, can solve problems such as complex curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1667] Preparation of phosphonate prodrugs
[1668] Prodrugs can be introduced at various stages of synthesis. Most frequently, these prodrugs are prepared from phosphonic acids of formula I (due to their stability).
[1669] Phosphonic acids of formula I can be alkylated under nucleophilic substitution conditions with electrophiles such as alkyl halides and alkyl sulfonates to give phosphonates. For example, where YR 11 Compounds of formula I which are acyloxyalkyl groups can be dissolved in a suitable solvent such as DMF (J.Med.Chem.37:1875 (1994)) in a suitable base (for example, pyridine, TEA, diisopropylethylamine ) in the presence of an appropriate acyloxyalkyl halide (eg, Cl, Br, I; Phosphorus Sulfur 54:143 (1990); Synthesis 62 (1988)) by direct alkylation of compounds of formula I. The carboxylate component of these acyloxyalkyl halides includes, but is not limited to, acetates, propionates, isobutyrates, valerates, benzoates, and other carboxylates.
[1670] Dimet...
example 1
[1797] Compound 1: N-[3,5-Dimethyl-4-(3′-isopropyl-4′-hydroxyphenoxy)]carbamoylphosphonic acid
[1798]
[1799] Step a:
[1800] 3,5-Dimethyl-4-(3'-isopropyl-4'-methoxyphenoxy)aniline (J.Med.Chem.38: 695 (1995), 0.1 g, 0.35 mmol) and diphosgene (0.04 g, 0.19 mmol) were heated at 60° C. for 3 h. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. To the residue was added a solution of phosphite (0.06 g, 0.42 mmol) in hexane (1.0 mL, added 3 drops of triethylamine), and the reaction mixture was heated at reflux for 3 h. The reaction mixture was cooled to room temperature and the solvent was removed under reduced pressure. The crude product was purified by column chromatography on silica gel, eluting with ethyl acetate-hexane (1:3), to obtain diethylphosphonate (0.1 g, 64%) as an oil: 1 H NMR (300MHz, CDCl 3 ): δ8.44(s, 1H), 7.17(s, 2H), 6.10-6.60(m, 3H), 4.10(m, 4H), 3.58(s, 3H), 3.07(m, 1H), 1.92( s, 3H), 1.93 (s, 3H...
example 2
[1804] Compound 2: 1-amino-2-[3,5-diiodo-4-(4'-hydroxy-3'-iodophenoxy)phenyl]ethylphosphonic acid
[1805]
[1806] Step a:
[1807]To 4-benzyloxyphenylacetyl chloride (4.0 g, 16.2 mmol) in THF (10.0 mL) was slowly added triethyl phosphite (3.33 mL, 19.5 mmol) at room temperature. The reaction mixture was stirred at room temperature for 16 h and the solvent was removed under reduced pressure. The residue was treated with hexane (20 mL) and the mixture was filtered. The white solid was collected and air dried. The solid was dissolved in pyridine (25.0 mL) and hydroxylamine hydrochloride (1.96 g, 28 mmol) was added. The reaction mixture was stirred at room temperature for 72 h, and the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography eluting with ethyl acetate-hexane (7:3) to obtain 2-(4-benzyloxyphenyl)-1-(hydroxyimino) as colorless oil Diethyl ethyl phosphonate (5.2 g, 85%): 1 H NMR (300MHz, CDCl 3 ): δ7.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com