1 typerecombinant adeno related viral vaccine of HPV16 and method of producing the same
A virus vaccine and virus technology, applied in the field of HPV16 type 1 recombinant adeno-associated virus vaccine and its preparation, can solve the problems of low expression level of L1 protein, and achieve the effect of increased titer and good preventive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0018] Experimental Example 1: Construction of recombinant adeno-associated virus containing the nucleic acid sequence shown in SEQ ID No: 1
[0019] 1. Construction of type I recombinant adeno-associated virus shuttle plasmid containing the nucleotide sequence shown in SEQ ID No: 1
[0020] (1) Preparation of Escherichia coli DH5α competent cells:
[0021] Pick a single colony of DH5α from a fresh plate cultured at 37°C for 16-20 hours, inoculate it in 5 ml of LB medium without antibiotics, and culture it overnight (12-16 hours) at 37°C with vigorous shaking. On the next day, draw 0.5ml from the above-mentioned culture and transfer it to 50ml LB medium at a ratio of 1:100 to continue culturing for about 3h. When the OD600 value of the bacterial solution is 3, transfer the bacteria to a sterile, Place in an ice-cooled 50ml centrifuge tube for 30 minutes. Centrifuge at 4000rpm for 10min at 4°C, discard the supernatant, invert the tube for 1min to drain the residual culture so...
experiment example 2
[0042] Experimental example 2: Identification of recombinant adeno-associated virus rAAV-mod.HPV16L1, virus titer analysis and electron microscope observation
[0043] (1) Expression of mod.HPV16L1 gene in recombinant adeno-associated virus:
[0044] Infect 1×10 with 10 MOI of rAAV-mod.HPV16L1 virus 6 293 cells, add sodium butyrate with a final concentration of 10mmol / L at the same time, scrape off the cells after 48 hours, wash 2 times with ice-cold PBS, extract the total protein of cells according to the instructions of TRIzol reagent, dissolve in 1% SDS solution, 0.1% SDS dialyzed 3 times, centrifuged at 10,000 g for 10 min at 4°C, and collected the supernatant. For the total protein content of BCA cells, adjust the protein concentration to 5 mg / ml. The total cell protein extracted with 50 μg TRIzol was used for SDS-PAGE electrophoresis and transferred to membrane. Mouse anti-HPV 16L1 monoclonal antibody (camvir-1) was used as the primary antibody, and horseradish enzyme...
experiment example 3
[0049] Experimental example 3: Preliminary study on the immune effect of recombinant adeno-associated virus rAAV-mod.HPV16L1:
[0050] 1. Immunization of Mice
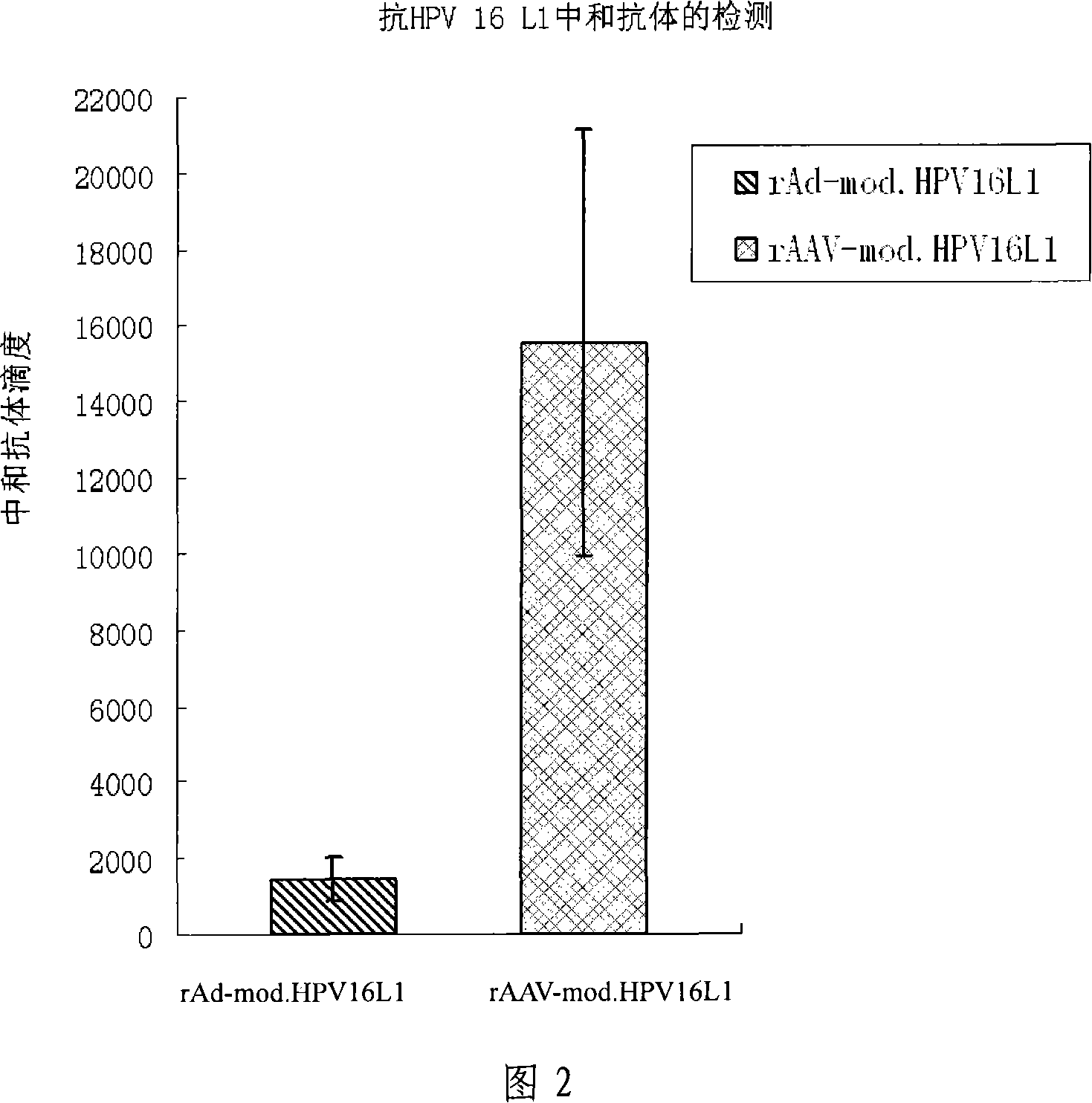
[0051] Twenty 4- to 6-week-old female C57BL / 6 mice were randomly divided into 2 experimental groups, 5 mice in each group, respectively rAAV-mod.HPV16L1 group; Recombinant adenovirus rAd-mod.HPV16L1. Each experimental group was set up with corresponding rAAV-EGFP and rAd-EGFP control groups according to the route of inoculation (the control viruses were all purchased from Yuanyuan Zhengyang Gene Technology Co., Ltd.). The doses used were rAAV 1×10 11 vg / piece; rAd 1×10 7 IU / rat, injected into the tibialis anterior muscle of both sides, each group was immunized once. All experimental operations were performed under anesthesia in mice. At 12 weeks after immunization, the serum and vaginal secretions of mice in each group were collected for future use.
[0052] 2. Detection of anti-HPV 16L1 neutralizing antibody
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com