2-vinyl-8-hydroxyquinoline derivative, preparation method and application thereof
A hydroxyquinoline and vinyl technology, which is applied in the field of preparation of organic compounds, can solve problems such as slow expansion speed, and achieve the effects of simple preparation, easy preparation and promotion of stem cell proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
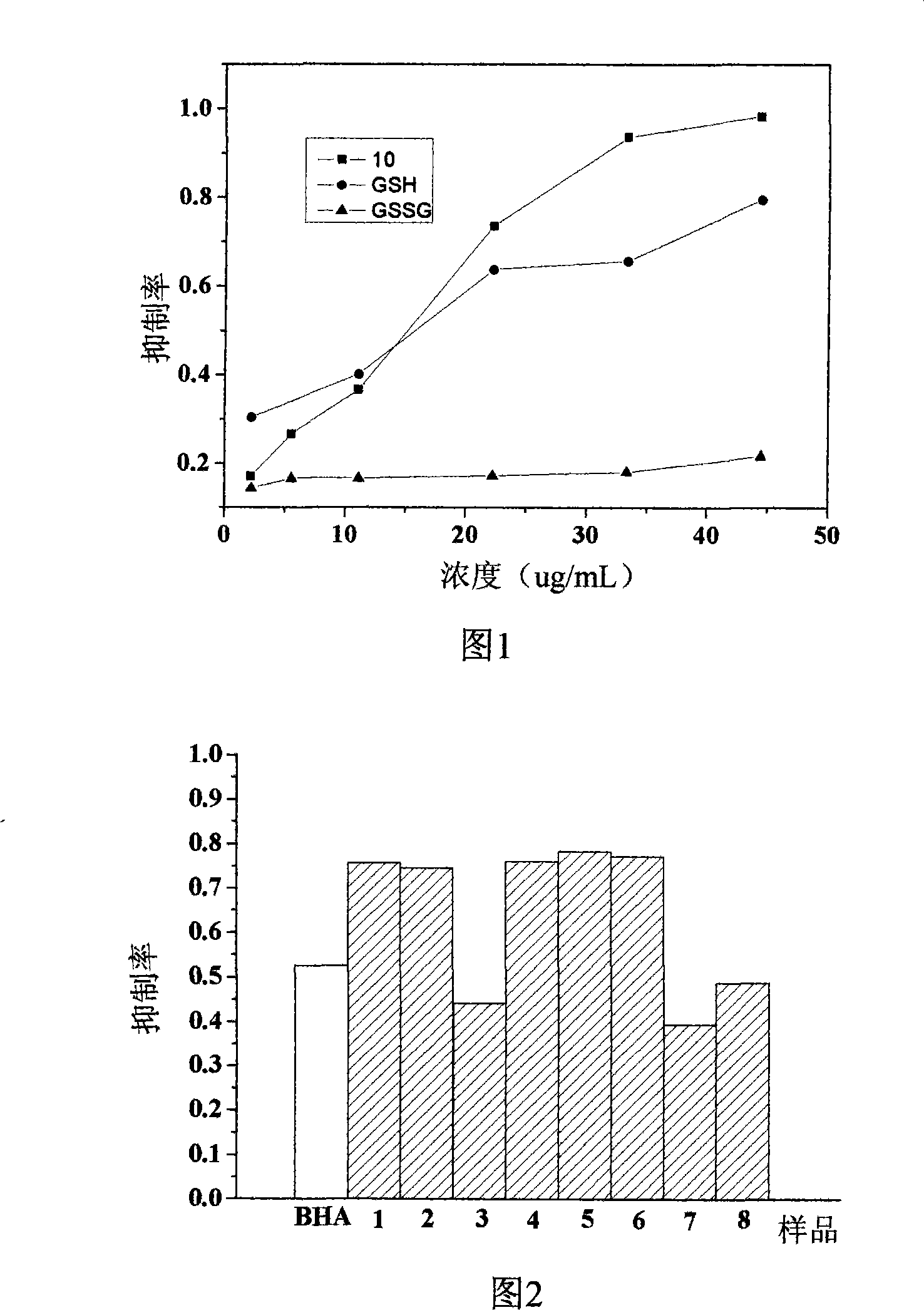
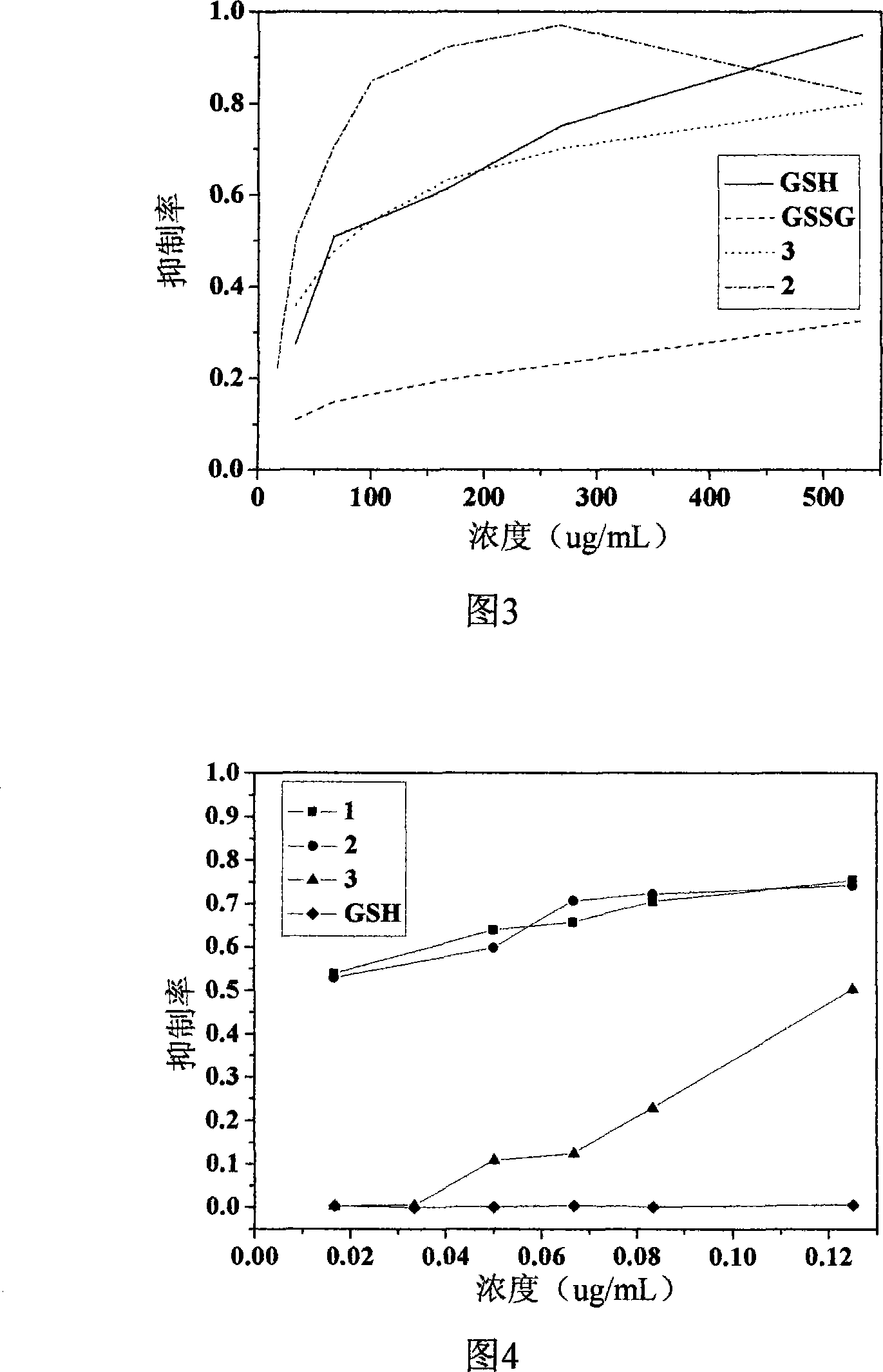
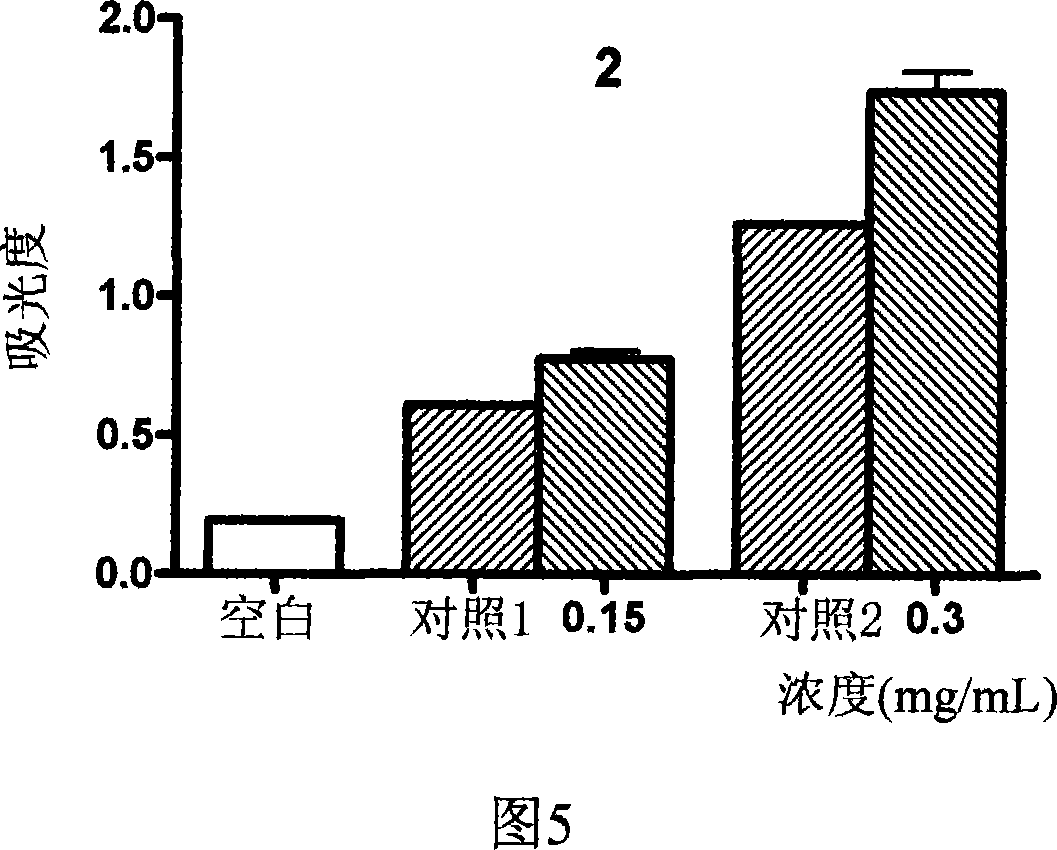
Image
Examples
Embodiment 1
[0026] Preparation of 2″-[2′-5-(2-methyl-8-hydroxyquinoline)-vinyl]-8″-hydroxyquinoline (Compound 1)
[0027] Weigh 2.340g 2-methyl-5-formyl-8-hydroxyquinoline and 1.790g 2-methyl-8-hydroxyquinoline, dissolve in 35mL acetic anhydride, stir to make it completely dissolved, in N 2 Under protection, heat and reflux for 40h at 130°C. During the reaction, the reaction was monitored by thin-layer chromatography. The reaction was completed and cooled to room temperature. Then the reaction mixture was poured into 50 mL of ice water, stirred for 3 to 4 hours, and the supernatant was poured out to obtain a brown-black viscous shape. After precipitation, silica gel column chromatography was performed using petroleum ether / ethyl acetate (V:V=9:1) as the eluent to obtain 1.325 g of the pure target product with a yield of 32.3%. m.p.187~189℃. Rf=0.45(V 石油醚 / V 乙酸乙酯 =3:1). ESI-MS m / z: 329.4 (M+H). IR(KBr)v(cm -1 ): 3410.37, 2925.50, 1631.78, 1592.55, 1501.37, 1462.00, 1202.20, 1181.52. 1 H NMR(CD...
Embodiment 2
[0029] Preparation of 2″-[2′-3-(1-hydro-indole)-vinyl]-8″-hydroxyquinoline (compound 2)
[0030] Weigh 0.795g 2-methyl-8-hydroxyquinoline and 0.725g 3-formylindole, dissolve in 20mL acetic anhydride, 2 Under protection, the reaction was refluxed with magnetic stirring at 125°C for 40h. After cooling, the reaction mixture was poured into 250 mL of ice water and stirred overnight. The resulting brown precipitate was washed with distilled water, and then petroleum ether / ethyl acetate (V:V=8:1) was used as eluent for silica gel column layer Analysis and separation yielded 0.61 g of 2"-[2'-3-(1-acetyl-indole)-vinyl]-8"-hydroxyquinoline acetate with a yield of 32.8%. m.p.194~196℃. UV(DMF)λmax: 306 nm, 361nm. 1 H NMR(CDCl 3 , 400MHz) δppm: 2.577 (s, 3H), 2.686 (s, 3H), 7.425 (m, 4H), 7.670 (m, 3H), 7.817 (d, J=16.4Hz, 1H), 7.958 (d, J =7.6 Hz, 1H), 8.134 (d, J=8.4 Hz, 1H), 8.483 (d, J=7.6 Hz, 1H). IR(KBr)v(cm -1 ): 3050.34 (CH stretching vibration on aromatic ring); 1746.05, 1641.81 (C=O...
Embodiment 3
[0033] Preparation of 2″-[2′-2-(1-methyl-benzimidazole)-vinyl]-8″-hydroxyquinoline (Compound 3)
[0034] 0.795 g 2-methyl-8-hydroxyquinoline and 0.80 g 1-methyl-2-formyl-benzimidazole were dissolved in 20 ml of acetic anhydride, and under the protection of nitrogen, the reaction was refluxed with magnetic stirring at 130° C. for 48 h. After cooling, pour into 250ml of ice water and stir overnight to obtain a yellow precipitate. After washing with distilled water, perform silica gel column chromatography with petroleum ether / ethyl acetate (V:V=3:1) as the eluent to obtain the target product 0.692g , The yield is 46%. m.p.207~208℃. UV(DMF) λmax: 321nm, 367nm. 1 H NMR (CDCl3, 400MHz) δppm: 8.183 (d, J = 8.4 Hz, 1H), 8.128 (d, J = 15.2 Hz, 1H), 7.890 (d, J = 16.0 Hz, 1H), 7.817 (m, 1H) ), 7.618(d, J=8.4Hz, 1H), 7.455(t, J=7.6Hz, 1H), 7.392(m, 1H), 7.333(m, 3H), 7.207(d, J=8.8Hz, 1H) ), 1.591 (S, 3H). IR(KBr)v(cm -1 ): 34 07.75 (O-H stretching vibration); 1637.87 (C=C bond stretching v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com