Separation method for Leydig cell and use thereof
A cell and stem cell technology, applied in the field of separation of cells in the Leydig, can solve the problems of long duration, low acquisition rate, and difficulty in meeting the number and function of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
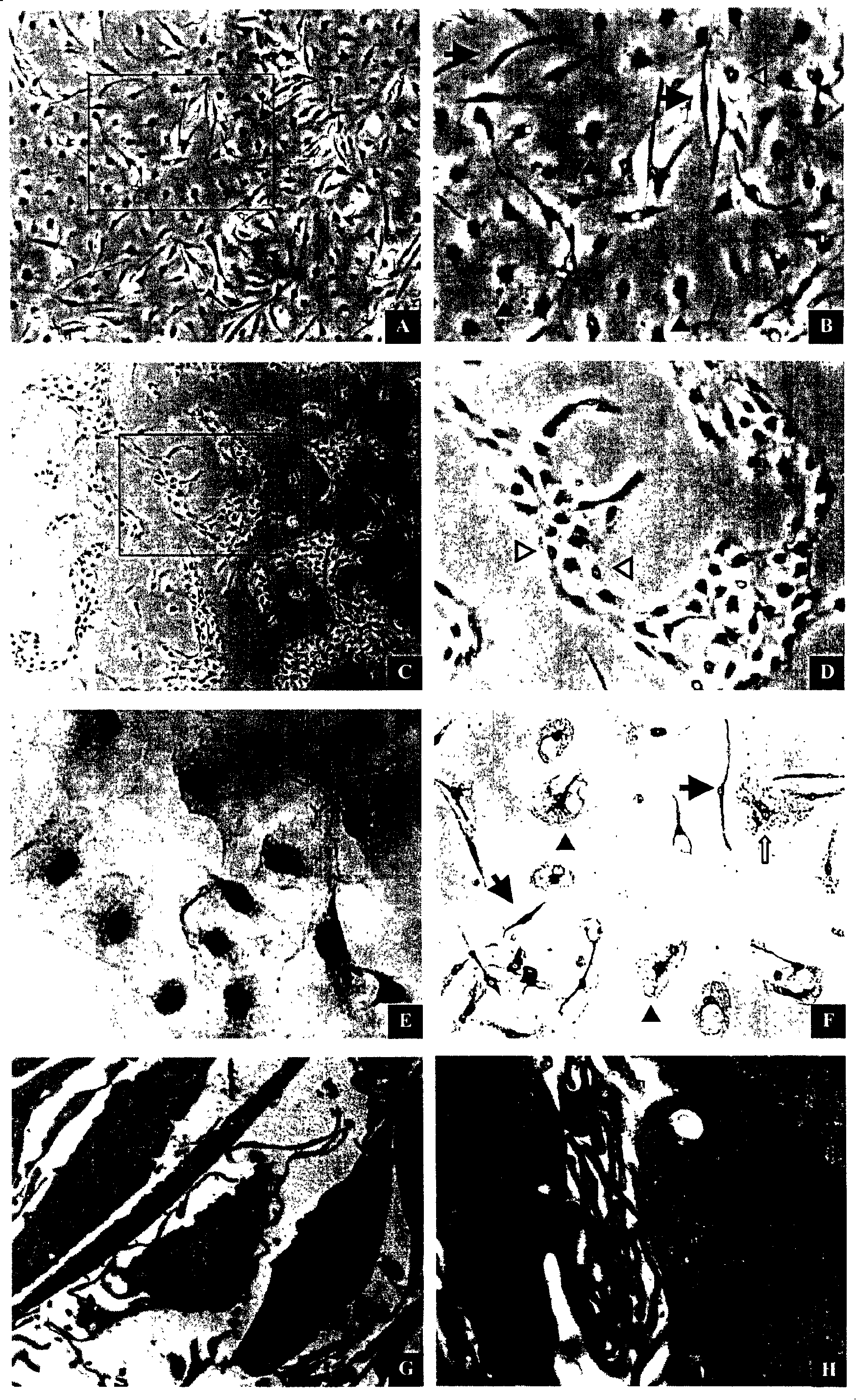
[0104] Acquisition, culture and detection of Leydig cells
[0105] 1. Obtaining Testicular Tissue
[0106] Male Wister rats, 30-45 days old, weighing 100-200 grams, were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0107] 2.5% pentobarbital anesthesia, bilateral inguinal canal medial oblique incision, cut the testicular sheath, carefully separate the connection between the testis and epididymis, obtain a complete testis after ligation of blood vessels, aseptically weigh the wet weight of each testis, That is, place in a sterile ice bath in phosphate buffered saline (PBS) without calcium and magnesium ions. The complete testis was obtained by repeated washing, and the outer layer of the testis and large blood vessels were peeled off on ice to keep the integrity of the testis shape and the continuity of the seminiferous tubules as much as possible. After removing the buffy coat and larger blood vessels visible to the naked eye, the wet wei...
Embodiment 2
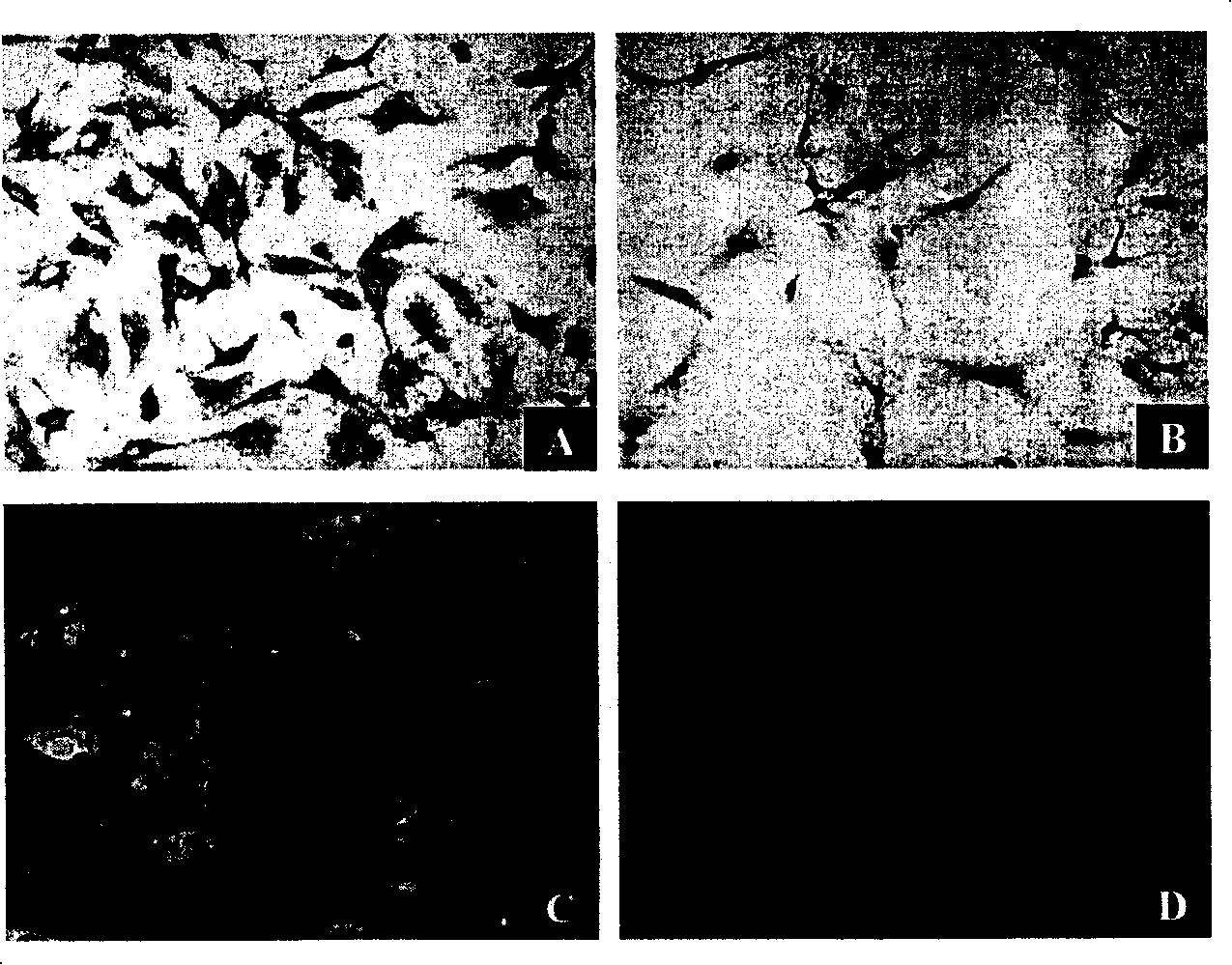
[0140] Differential attachment time selection
[0141] According to the method of Example 1, cells in the testicular tissue and interstitial tissue of the testis were obtained, filtered and then inoculated and cultured.
[0142] The cultured cells were divided into four groups as follows: (1) 1-hour group; (2) 2-hour group; (3) 3-hour group and (4) 24-hour group.
[0143] The specific operation is as follows: After inoculation, after culturing for 1, 2, 3 and 24 hours according to the groups, the unattached suspension cells and culture fluid were collected respectively, and transferred to the corresponding new culture dishes of each group, with 5 % serum in DMEM / HAM'S F-12 (1:1) culture solution, after 24 hours, remove unattached cells and culture solution. At the same time, the adherent cells in the original culture dish of each group were added with serum-free DMEM / HAM'S F-12 (1:1) medium to continue culturing.
[0144] If the cells cultured after transfer do not re-attach t...
Embodiment 3
[0147] Optimization of Conditioned Medium for Leydig Cells
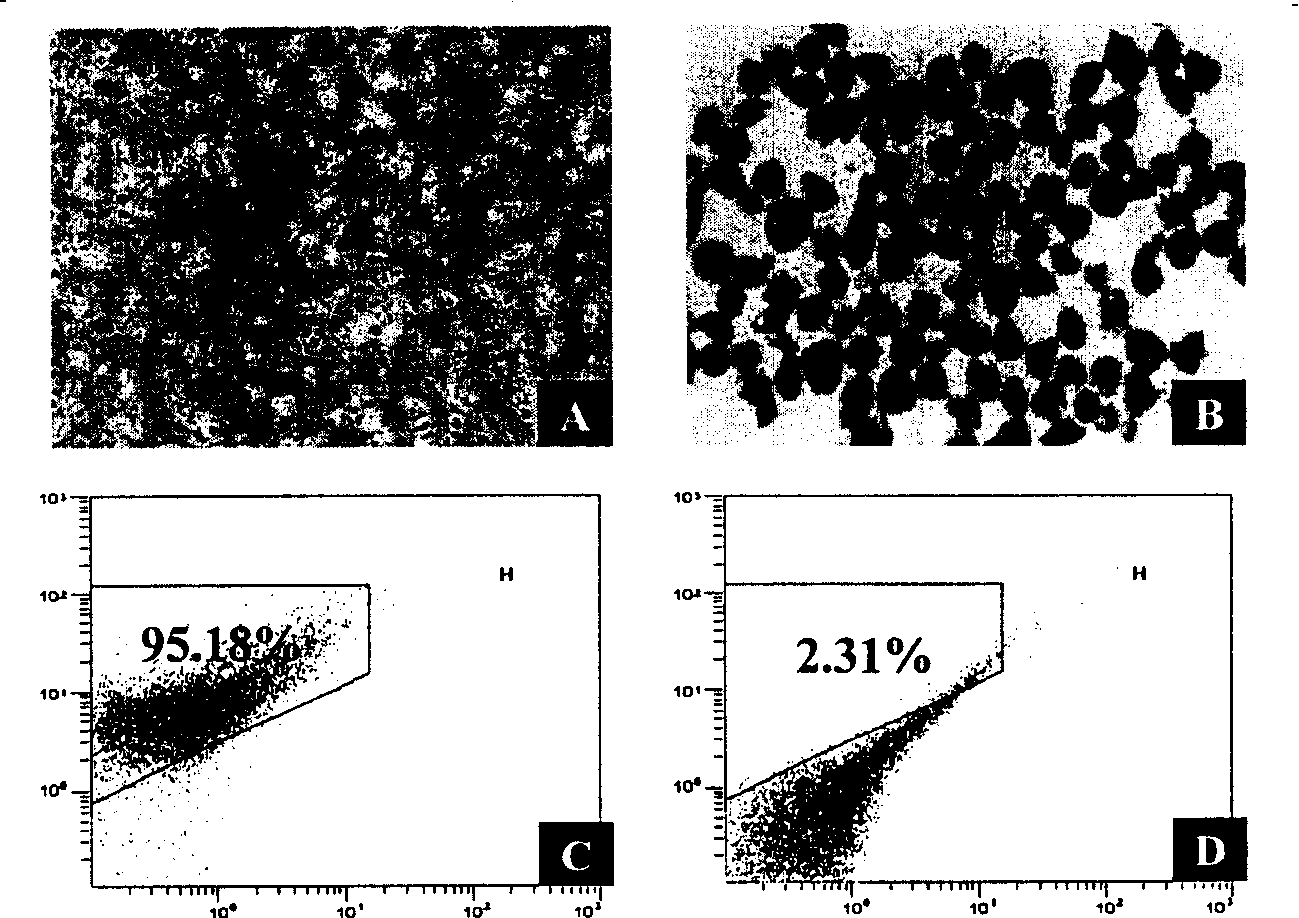
[0148] According to the method of Example 1, cells in the testicular tissue and interstitial tissue of the testis were obtained, filtered and then inoculated and cultured. The DMEM / HAM'S F-12 (1:1) culture medium for cell primary culture was divided into the following four groups: (1) serum-free group; (2) group added with 1% fetal bovine serum; (3) group added with 5% Fetal bovine serum group; and (4) adding 10% fetal bovine serum group. Then the cell morphology and growth characteristics were observed under an inverted fluorescent microscope and an electron microscope.
[0149] See Figure 5 .
[0150] The results show that the primary cells can maintain good cell growth morphology for more than 7 days when cultured under the condition of serum-free DMEM / HAM'S F-12 (1:1), and the DMEM / HAM'SF containing 1% fetal bovine serum -12 (1:1) culture medium can accelerate the spread and growth speed of early Leydig cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com