Non-aqueous electrolyte and Li-ion secondary battery, and method for producing them
A non-aqueous electrolyte, electrolyte technology, applied in the direction of non-aqueous electrolyte storage battery, electrolyte storage battery manufacturing, secondary battery, etc., can solve problems affecting battery performance, achieve good high-temperature storage performance, increase discharge capacity at room temperature, preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
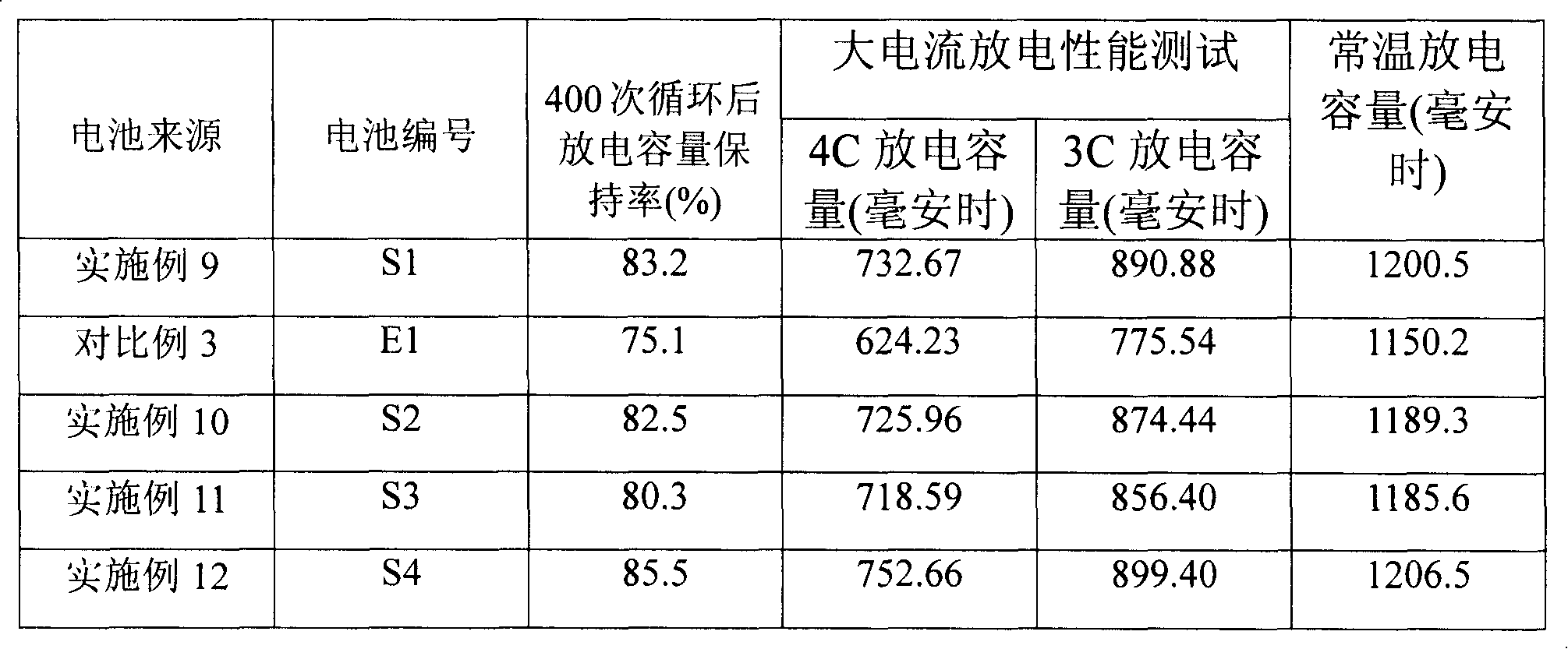
Examples
preparation example Construction
[0018] The preparation method of the electrolyte solution of the present invention is a routinely used method. It only needs to dissolve the lithium salt and the weak salt of lithium in an organic solvent. The concentration of the lithium salt as electrolyte is 0.5-2.0 mol / liter, preferably 0.5-1.5 mol / liter. Based on the total amount of the non-aqueous electrolyte, the content of the weak salt of lithium is 0.001-5% by weight, preferably 0.005-3% by weight.
[0019] Preferably, the weak acid salt of lithium is pre-baked at 120-180° C. for 5-24 hours before being mixed with the electrolyte, so that the water content of the weak acid salt of lithium is below 30 ppm. In order to fully and efficiently remove a very small amount of moisture in the weak salt of lithium, the baking is preferably performed in a vacuum oven, and the vacuum pressure of the vacuum oven is less than atmospheric pressure.
[0020] The lithium ion secondary battery of the present invention includes an el...
Embodiment 1
[0037] This example illustrates the non-aqueous electrolyte provided by the present invention and its preparation method.
[0038] Mix 100 ml of ethylene carbonate, 100 ml of diethyl carbonate and 100 ml of ethyl methyl carbonate, add 45.573 g of LiPF as electrolyte lithium salt 6 , stir evenly to obtain a mixed solution with an electrolyte lithium salt concentration of 1.0 mol / liter, then add 3.756 grams of lithium acetate as a weak salt of lithium, based on the total amount of the electrolyte, the content of the weak salt of lithium 1.0% by weight, and stirred evenly to obtain electrolyte solution A1.
Embodiment 2
[0042] The preparation of the electrolyte is carried out according to the method of Example 1. The difference is that the weak acid salt of lithium is lithium benzoate, and the consumption is 11.267 grams. Based on the total amount of the electrolyte, the amount of the weak acid salt of lithium is The content was 3.0% by weight to obtain electrolytic solution A2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com