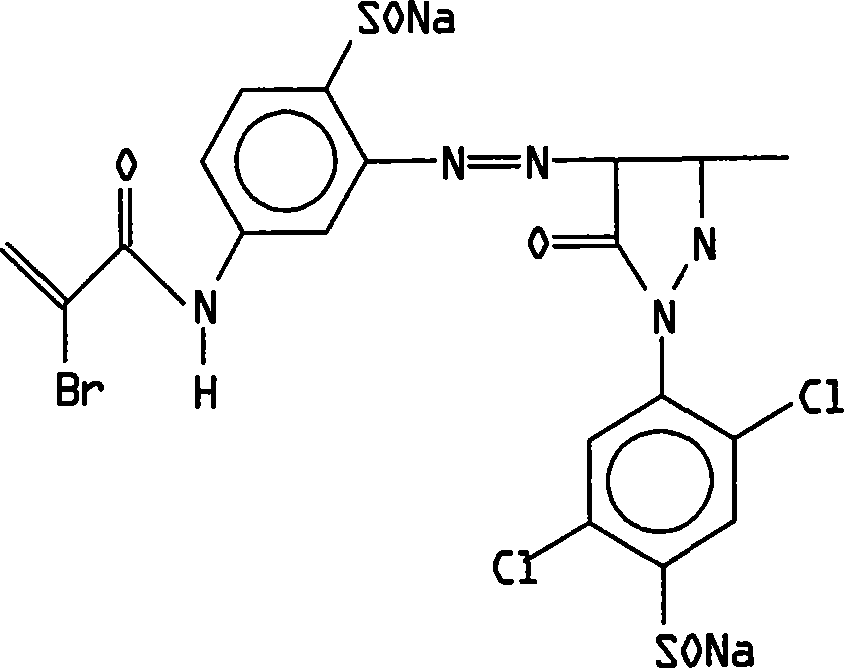
Method of preparing yellow active dyestuff
A reactive dye and yellow technology, which is applied in the field of preparation of yellow reactive dyes, can solve the problems of low color yield, large consumption of raw materials, dust pollution, etc., and achieve the effects of good reproducibility, improved solubility and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
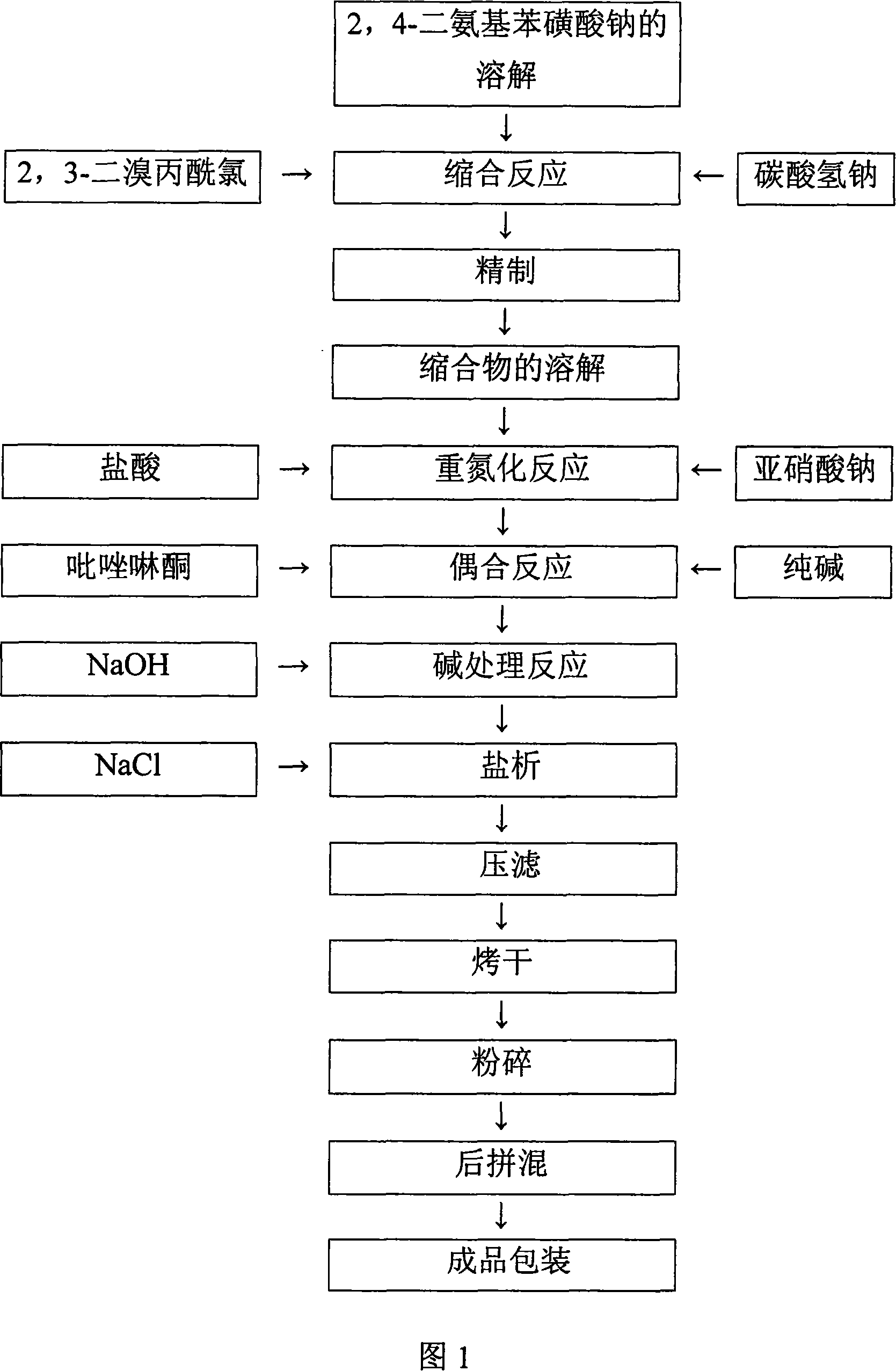
[0025] As shown in Figure 2, the preparation process of the yellow reactive dye of the present invention, a preparation method of the yellow reactive dye, comprises the following steps in turn:
[0026] a. Condensation reaction: prepare an aqueous solution of sodium 2,4-diaminobenzenesulfonate with a pH of 6 to 6.5 with a mass percentage concentration of 10-11%, add 2,3-dibromopropionyl chloride dropwise at a low temperature, stir, and react pH=6~6.5, until the reaction is complete;
[0027] b. Diazotization reaction: add the hydrochloric acid solution to step a, adjust the volume with water to make the mass percentage concentration of the solution 7%, ensure that the Congo red test paper is blue after dipping, and the KI test paper is slightly dipped after dipping. Under the blue condition, add sodium nitrite solution dropwise until the reaction is complete;
[0028] c. Coupling reaction: eliminate the excess nitrous acid in step b with sulfamic acid, add water to adjust the...
Embodiment 1
[0041] 1. Ingredients list:
[0042] raw material name
molecular weight
Molecular ratio
100% dosage (Kg)
Feeding amount (kmol)
Sodium 2,4-diaminobenzenesulfonate
2,3-Dibromopropionyl chloride
1-(2,5-dichloro-4-sulfonic acid)benzene
yl-3-methyl-5-pyrazolone
210
250.5
69
36.5
323
1
1.05
0.816
2
0.8
288.75
361.66
77.42
100.38
355.3
1.375
1.375
1.375
1.375
1.375
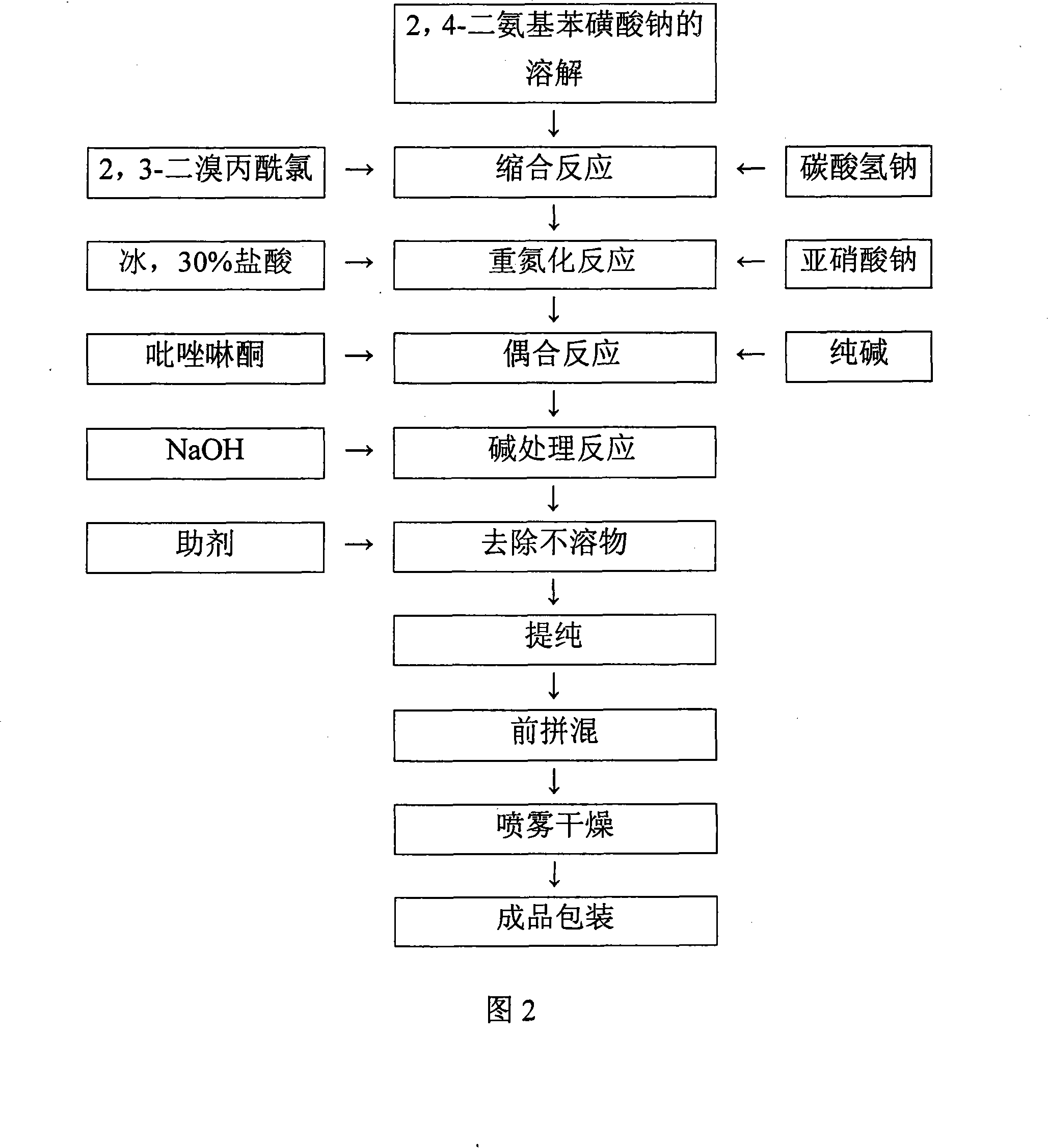
[0043] 2, the yellow reactive dye preparation process of the present invention operates as follows:
[0044] a. Dissolution of sodium 2,4-diaminobenzenesulfonate
[0045] Put 2400 liters of bottom water into the reaction tank, add 288.75 kg of sodium 2,4-diaminobenzenesulfonate, heat up to 22°C, stir to dissolve, and make the material completely dissolved and clarified. Adjust pH=...
Embodiment 2
[0077] a. Dissolution of sodium 2,4-diaminobenzenesulfonate
[0078] Put 2400 liters of bottom water into the reaction tank, add 288.75 kg of sodium 2,4-diaminobenzenesulfonate, heat up to 22°C, stir to dissolve, and make the material completely dissolved and clarified. Adjust pH=6~6.5 with dilute hydrochloric acid. The volume is 2625 liters, and the mass percentage concentration of the solution is 11%.
[0079] b. Condensation reaction
[0080] Directly use ice and jacketed circulating cold water to adjust the temperature of solution a to 0 to 5 °C, adjust the mass percentage concentration of the solution to 10%, add 2,3-dibromopropionyl chloride within 1.5 to 2 hours, and use sodium bicarbonate at the same time. The pH was maintained at 6-6.5, and the reaction was stirred for 2 hours. Reaction temperature 0~5 ℃, reaction pH=6~
[0081] 6.5. Finally, the end point was detected by TLC aluminum-based silica gel thin layer plate.
[0082] c, diazotization reaction
[0083...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com