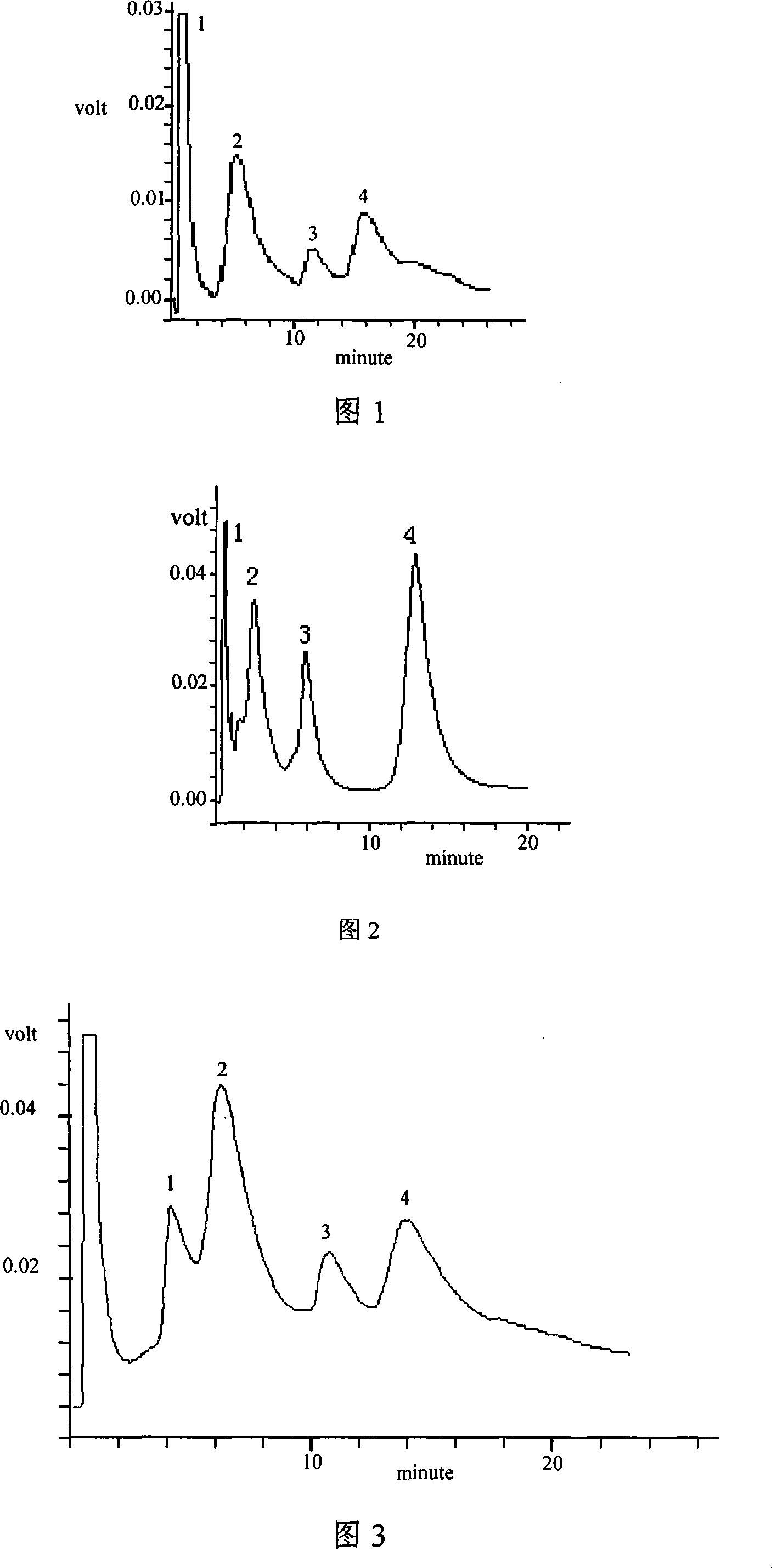
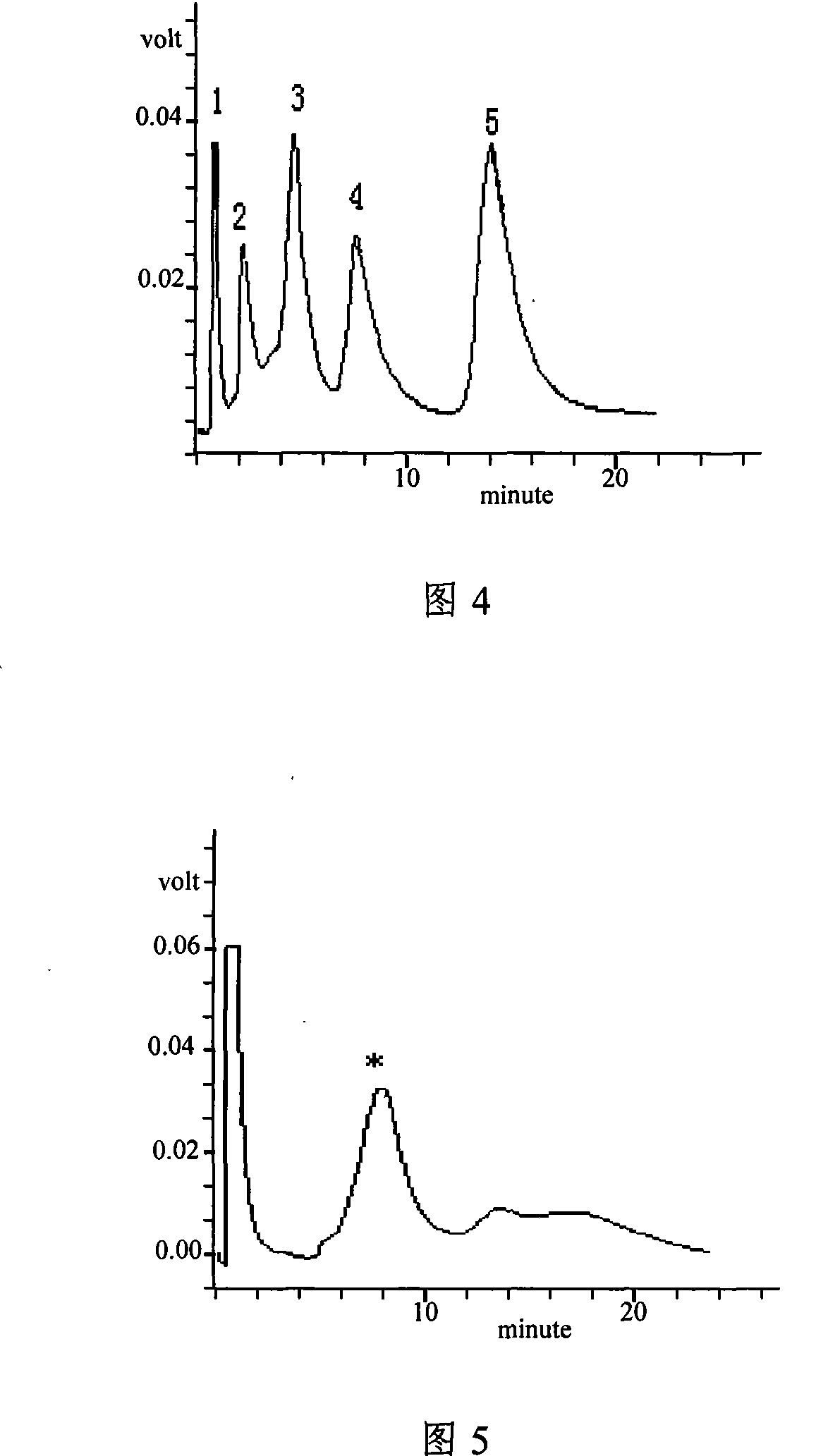
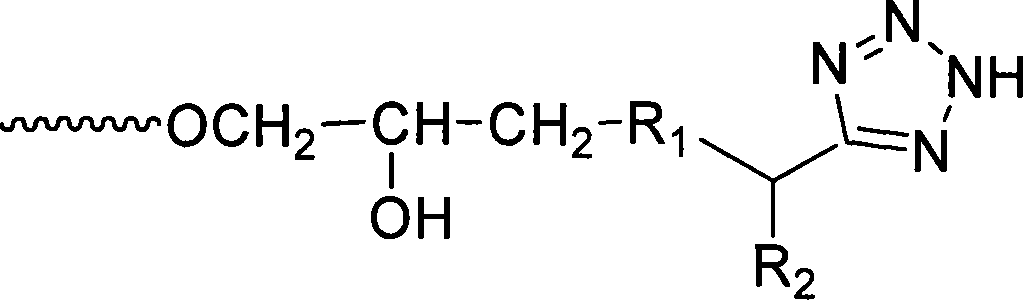Multifunctional separation medium with tetrazole as functional group and preparing method thereof
A separation medium and multi-purpose technology, applied in the field of chromatographic separation, can solve the problems of metal ion loss and single use, and achieve the effect of fast separation speed, high purity and easy regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] (1) Preparation of α-aminotetrazole (R 2 Optional CH 2 CH(CH 3 ) 2 , CH(CH 3 ) 2 , CH 3 , C 6 h 5 , CH(CH 3 )CH 2 CH 3 or CH 2 C 6 h 5 )
[0036]
[0037] Synthetic route of α-aminotetrazole
[0038]Using Fmoc-protected leucine, isoleucine, valine, alanine and phenylalanine as starting materials, according to the literature method (Huo Yanmin, Lei Genhu, Zheng Xiaohui, Wei Yinmao.Synthesis and characterization of the tetrazole analogues of α-amino acids. Chinese Chemical Letters, 2006, 17(12), 1537-1539), respectively prepare Fmoc-protected α-amino tetrazole. Add Fmoc-protected α-aminotetrazole (2.5 mmol), 100 mL of 20%-50% triethylamine in methanol solution to a 250 mL round bottom flask, and stir at room temperature for 30 min. After the reaction was completed, the solvent was evaporated, and an appropriate amount of 0.5mol L was added -1 Hydrochloric acid until no solid is dissolved, extract the insoluble matter with petroleum ...
Embodiment 1
[0043] Embodiment 1: α-aminotetrazole is the preparation of the silica gel separation medium of ligand
[0044]
[0045] Preparation route of silica gel separation medium with α-aminotetrazole as ligand
[0046] Weigh 2 grams of macroporous silica gel, add 30 mL of 1:1 hydrochloric acid, ultrasonicate for 5 minutes, and then reflux in an oil bath at 100°C for 3 hours. After the reaction is complete, filter, wash with distilled water until neutral, and dry at 130°C for 4 hours. Add the silica gel into a 100mL three-neck flask, add 35mL of dry toluene, ultrasonicate for 5min, and after the temperature rises to 120°C, add 1.8mL of γ-glycidyl etheroxypropyltriethoxysilane dropwise with a dropping funnel under stirring, Reflux for 12 hours. After the reaction is completed, filter, wash with dry toluene 3 times, and wash with acetone 4 times. Vacuum dry at 50°C for later use.
[0047] Add 30mL DMF, 0.5g α-aminophenylpropane tetrazole and 0.1mL pyridine to the dry epo...
Embodiment 2
[0048] Embodiment 2: α-aminotetrazole is the preparation of the agarose (or dextran) separation medium of ligand
[0049]
[0050] α-Aminotetrazole is the preparation route of the agarose (or dextran) separation medium of the ligand
[0051] Take 10 mL of agarose (or dextran) in a 100 mL three-neck flask, add 50 mL of 5% NaOH and 5.0 mL of epichlorohydrin, and react for 12 hours. After the reaction is completed, wash with phosphate buffer (pH 7.0) to obtain agarose (or dextran) with epoxy groups bound to the surface. In epoxy agarose (or dextran), add 50mL DMF, 1.5g α-aminophenylpropane tetrazole and 1.0mL pyridine, and stir at room temperature for 24h. After the reaction is completed, wash with phosphate buffer (pH 7.0) successively to obtain tetrazolium agarose (or dextran) chromatographic separation medium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com