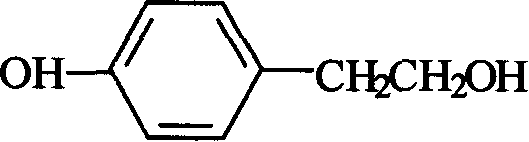
Method for synthesizing tyrosol
A technique for the synthesis of p-hydroxyphenylethanol and its synthesis method, which is applied in the field of synthesis of pharmaceutical intermediate p-hydroxyphenylethanol, can solve the problems of high anhydrous requirements, difficult separation, low yield, etc., and achieve environmental protection, low price, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) At 0°C, put 120ml (103g) of ethylene glycol dimethyl ether and 60g (0.36mol) of methyl p-hydroxyphenylacetate into the reactor, and stir for 15 minutes to completely dissolve the methyl p-hydroxyphenylacetate in ether.
[0021] (2) Add 15.2 g (0.4 mol) of sodium borohydride, gas is released, stir for 15 minutes, and keep at 0-10°C.
[0022] (3) At 0°C, add 80 ml of boron trifluoride diethyl ether dropwise, and the drop is completed in about 30 minutes. Continue stirring for 2 hours, add 300 ml of water, and stir for 30 minutes.
[0023] (4) After the reaction is completed, ethylene glycol dimethyl ether is recovered under reduced pressure. After adding 100ml of water, a large amount of white solids precipitated, and stirred for 15 to 20 minutes. Filter and wash the filter cake with ice water to obtain the product. Vacuum-dried to obtain 37 g of dry product, content (HPLC) ≥ 98.8%, mp: 88-92°C, yield 74.3%.
Embodiment 2
[0025] (1) At 0°C, put 120ml (103g) of ethylene glycol dimethyl ether and 64.8g (0.36mol) of ethyl p-hydroxyphenylacetate into the reactor, and stir for 15 minutes to completely dissolve the ethyl p-hydroxyphenylacetate in ether.
[0026] (2) Add 15.2 g (0.4 mol) of sodium borohydride, gas is released, stir for 15 minutes, and keep at 0-10°C.
[0027] (3) At 0°C, add 80 g of aluminum trichloride and finish adding in about 30 minutes, continue stirring for 2 hours, add 300 ml of water, and stir for 30 minutes.
[0028] (4) After the reaction is completed, ethylene glycol dimethyl ether is recovered under reduced pressure. After adding 200ml of water, a large amount of white solids were precipitated, and stirred for 15 to 20 minutes. Filter and wash the filter cake with ice water to obtain the product. Vacuum-dried to obtain 36 g of dry product, content (HPLC) ≥ 98.5%, mp: 88-92°C, yield 72.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com