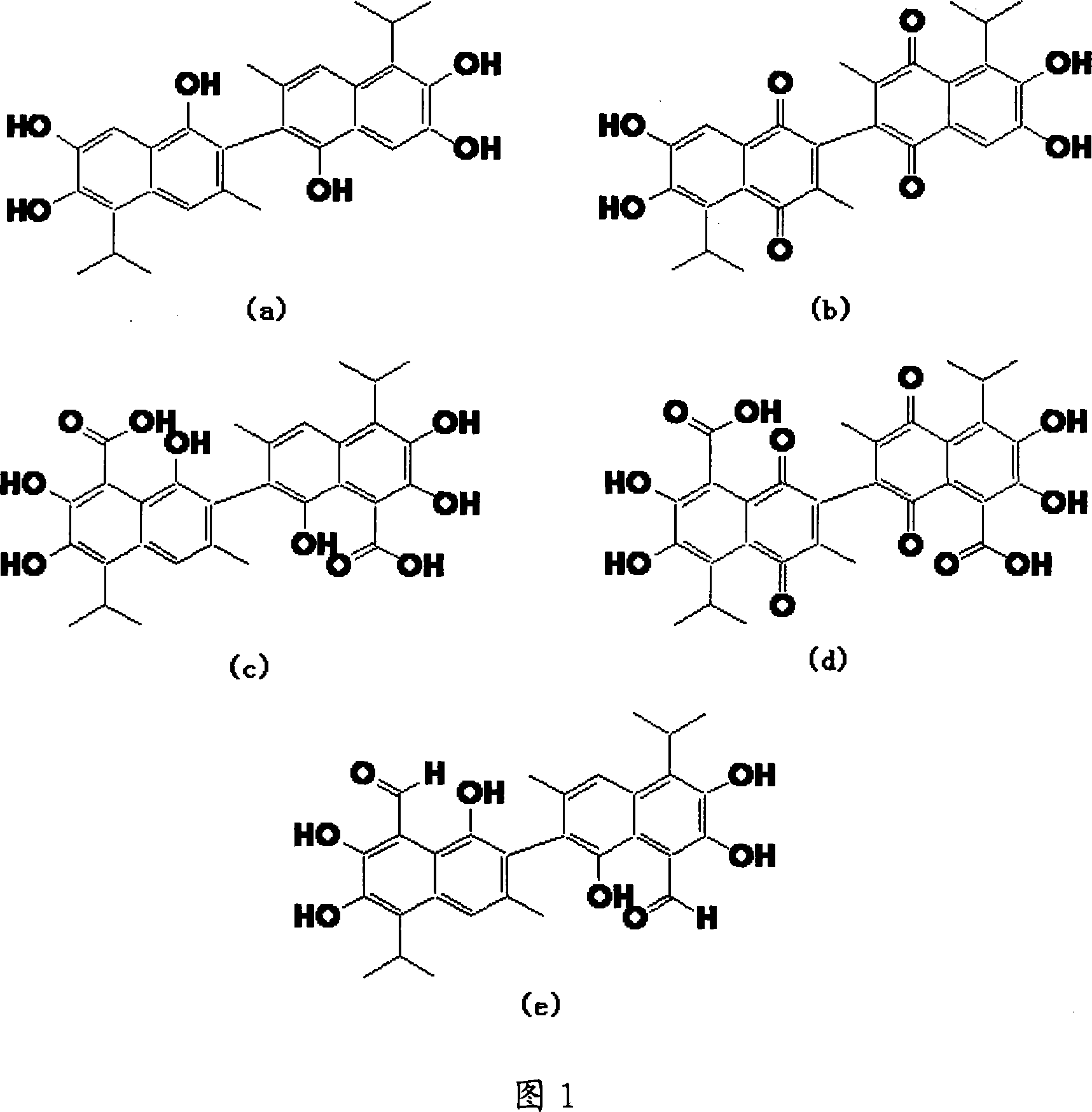
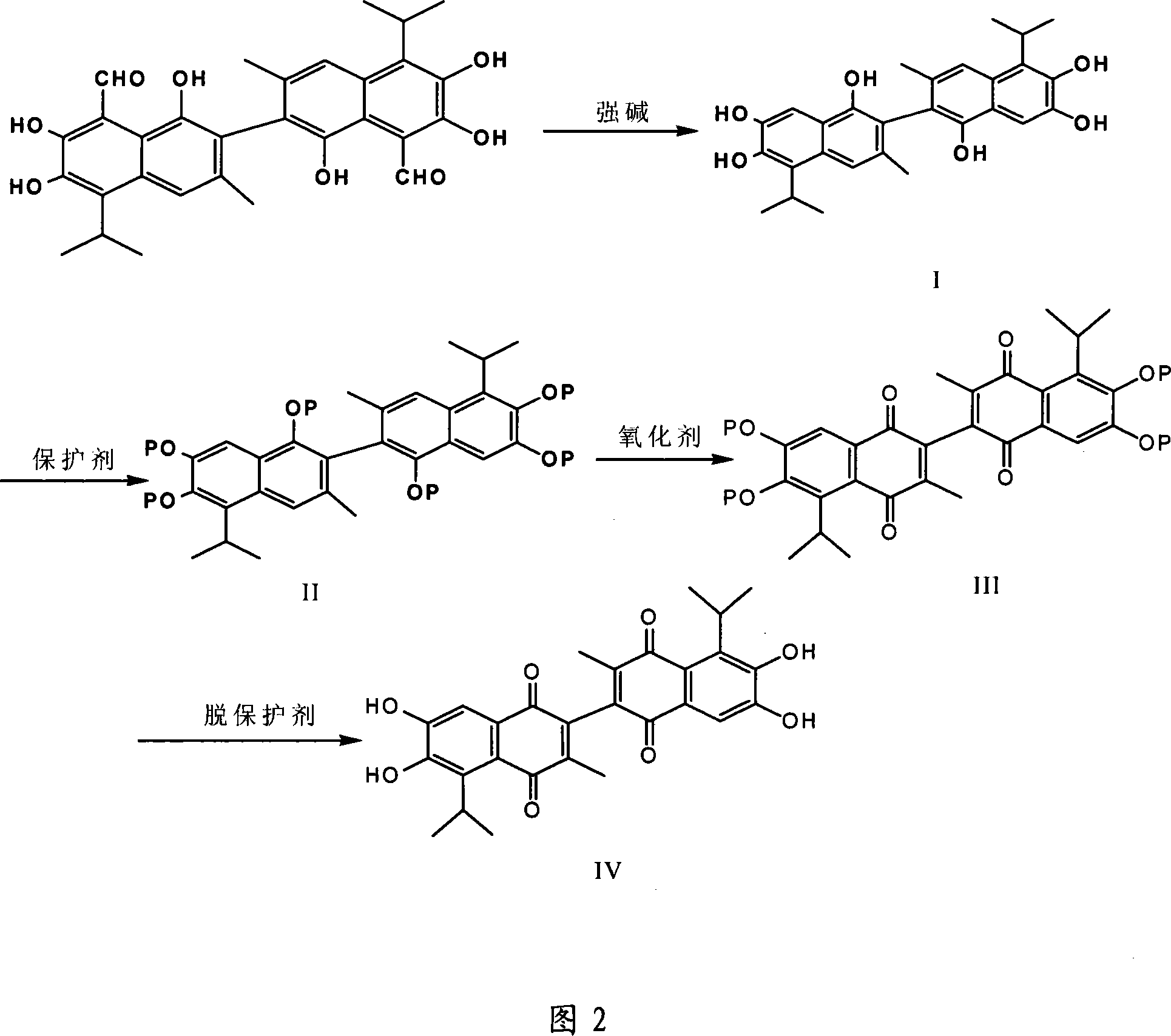
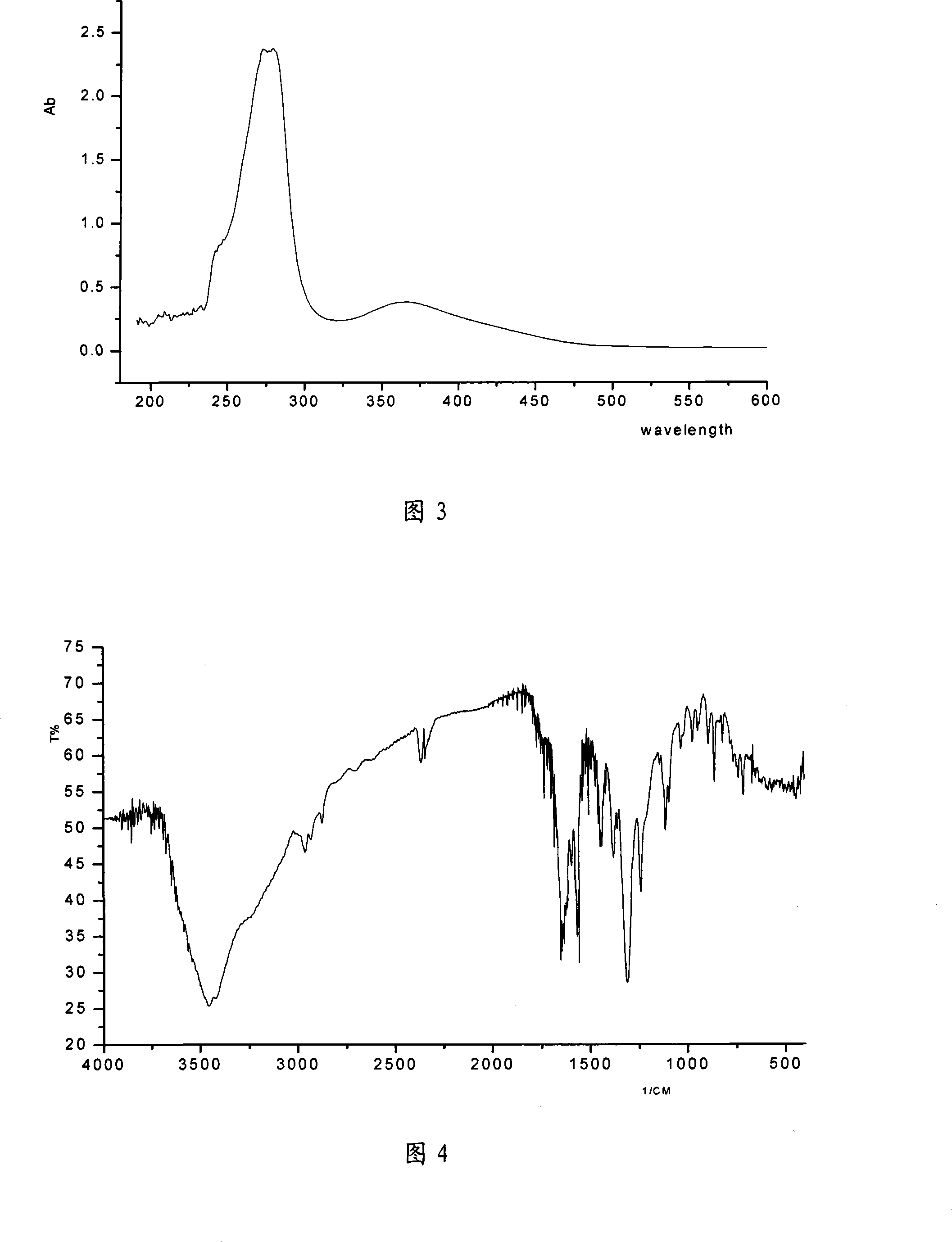
Method for synthesizing antitumor derivative apogossypolone by using gossypol acetate
An anti-tumor technology of gossypol acetate, applied in organic chemistry, quinone oxidation preparation, etc., to improve synthesis efficiency and yield, save time, and improve purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 5 grams of gossypol acetate with a balance, add 15 ml of 20% sodium hydroxide, and stir at 60°C for 300 minutes. After the reaction, place the reactor in ice water and add 15 ml of 85% concentrated sulfuric acid. Extract with diethyl ether two to three times, and concentrate to dryness in vacuo to obtain about 4.8 g of the initial product I, which is gray-green in color. Dissolve the initial product I with 9.6ml of dioxane, add 4.8ml of acetic anhydride, stir the reaction at 50°C for 120 minutes, cool to room temperature and let it stand for 30 minutes, extract 2-3 times with ethyl acetate, and concentrate in vacuo To dryness, recrystallization and purification with ethyl acetate / n-hexane (volume ratio 1:1) gave 3.6 g of pure product II with pale yellow color. Dissolve product II with 18ml of glacial acetic acid, add 3.6g of oxidant periodate, react at 60°C for 120 minutes, cool to room temperature and extract 2-3 times with ethyl acetate, concentrate to dryness u...
Embodiment 2
[0028] Weigh 5 grams of gossypol acetate with a balance, add 30 ml of 35% sodium hydroxide, and stir and react at 90°C for 165 minutes. After the reaction, place the reactor in ice water and add 7 ml of 90% concentrated sulfuric acid. Extracted two to three times with ethyl acetate, and concentrated to dryness in vacuo to obtain about 4.6 g of the initial product, which was gray-green in color. Dissolve the initial product I with 30ml of dioxane, add 15ml of acetic anhydride, stir the reaction at 120°C for 90 minutes, cool to room temperature after the end and leave it for 75 minutes, extract 2-3 times with ether, concentrate to dryness under vacuum, use Ethyl acetate / n-hexane (volume ratio 1:3) was used for recrystallization and purification to obtain 3.6 g of pure product II with pale yellow color. Dissolve the product II with 120ml of glacial acetic acid, add 20g of oxidant periodate, react at 90°C for 60 minutes, cool to room temperature after the end and extract 2-3 times...
Embodiment 3
[0030] Weigh 5 grams of gossypol acetate with a balance, add 50 ml of 50% sodium hydroxide, and stir and react at 120°C for 30 minutes. After the reaction, place the reactor in ice water and add 1 ml of 98% concentrated sulfuric acid. Extracted two to three times with petroleum ether, and concentrated to dryness in vacuo to obtain about 4.8 g of the initial product I, which was gray-green in color. Dissolve the initial product I with 48ml of dioxane, add 24ml of acetic anhydride, stir the reaction at 200°C for 20 minutes, cool to room temperature after the end and let it stand for 120 minutes, extract 2-3 times with ethanol, concentrate to dryness under vacuum, use Ethyl acetate / n-hexane (1:6) was used for recrystallization and purification to obtain 3.6 g of pure product II with pale yellow color. Dissolve the product II with 180ml of glacial acetic acid, add 36 grams of oxidant periodate, react at 150°C for 5 minutes, cool to room temperature after the end and extract with e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com