Cyclohexenone extract of antrodia camphorata
A technology of extracts and compounds, applied in the field of novel compounds, can solve problems such as the publication of active ingredients, and achieve the effect of avoiding cell mutation and preventing cardiovascular diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
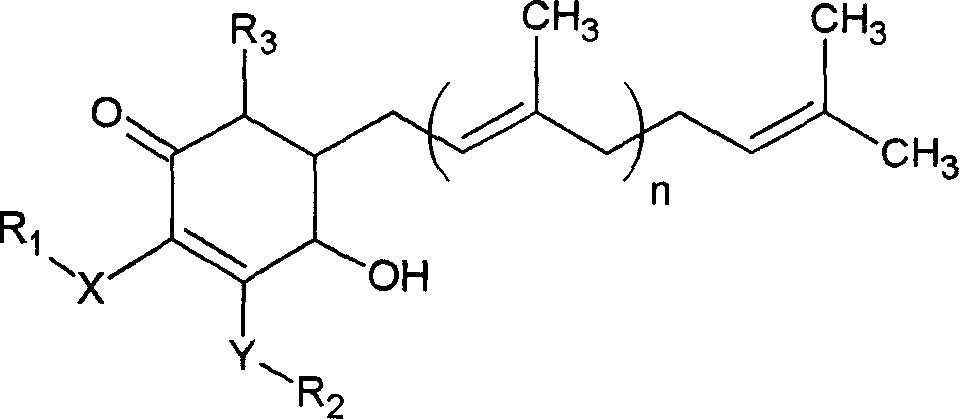
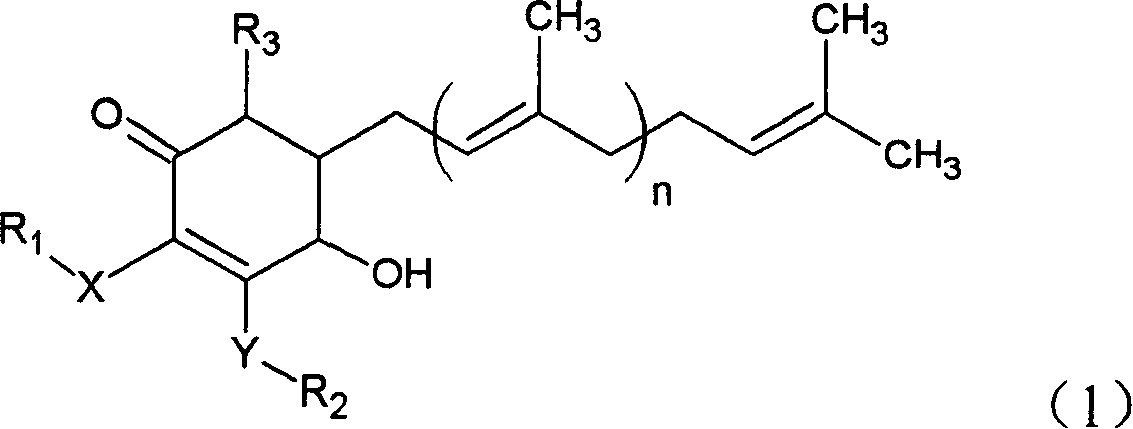
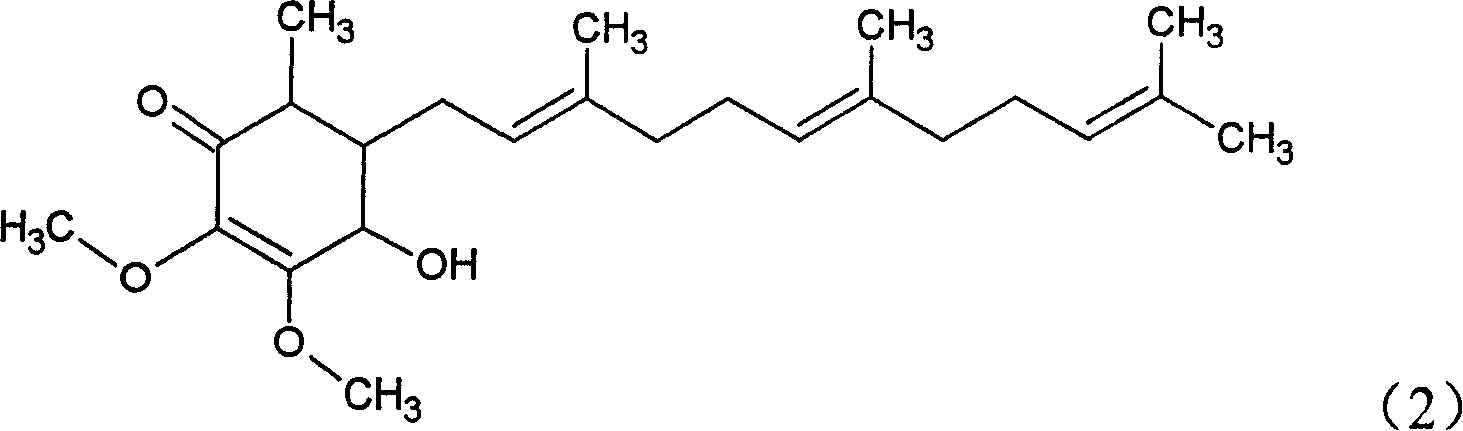
[0021] Example 1: 4-Hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-2,6,10-dodecatriene)-2-ring Separation of hexenone
[0022] Put about 100 grams of Antrodia antrodia mycelia, fruiting bodies or a mixture of the two into a conical flask, add an appropriate proportion of water and alcohol (70% to 100% alcohol aqueous solution), and heat it at 20 to 25 ° C. Stir and extract for at least 1 hour, then filter through filter paper and a 0.45 μm filter membrane to collect the extract.
[0023] The previously collected Antrodia camphorata extract was analyzed using a high performance liquid chromatography (High Performance Liquid chromatography) with an RP18 chromatographic tube (column), and methanol (A) and 0.1% to 0.5% acetic acid aqueous solution (B ) as the mobile phase (the solution ratio is: 0-10 minutes, B ratio is 95%-20%; 10-20 minutes, B ratio is 20%-10%; 20-35 minutes, B ratio 10% to 10%; 35 to 40 minutes, the proportion of B is 10% to 95%), eluting at a speed of 1ml...
Embodiment 2
[0026] Example 2: Activity test of anti-breast cancer tumor cells in vitro
[0027] In order to further test the inhibitory effect of the compound found in Example 1 on tumor cells, this example will first take the isolated compound in Example 1 according to the anti-tumor drug screening mode of the National Cancer Institute (NCI) in the United States. 4-Hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-2,6,10-dodecatriene)-2-cyclohexenone compound , adding MCF-7 and MDA-MB-231 human tumor cell culture medium to test the viability of tumor cells. The test of cell viability can be analyzed by the known MTT assay, and both MCF-7 and MDA-MB-231 are human breast cancer tumor cell lines.
[0028] MTT assay is a commonly used analytical method for analyzing cell proliferation, percent of viable cells, and cytotoxicity. Among them, MTT (3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide) is a yellow dye, which can be absorbed by living cells and absorbed by succinate tetra...
Embodiment 3
[0033] Example 3: Activity test for adjuvant therapy of breast cancer tumor cells in vitro
[0034] This test is also tested according to the National Cancer Institute's In Vitro Screening Model. First, human breast cancer cells MCF-7 and MDA-MB-231 were cultured in culture medium containing fetal calf serum for 24 hours, and the proliferated cells were washed once with PBS and washed with 1 times trypsin-EDTA Cells were treated followed by centrifugation at 1,200 rpm for 5 minutes to pellet the cells and discard the supernatant. Then add 10ml of new culture medium and shake gently to resuspend the cells. Before the test, first add 0.0017 μg / ml paclitaxel (Taxol) to treat the cells for 72 hours, then divide the cells into 96-well microplates, and then add 0 μg / ml (control group) to each well, 30, 10, 3 , 1, 0.3, 0.1 and 0.03 μg / ml of 4-hydroxyl-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-2, 6,10-dodecatriene)-2-cyclohexenone (test group), at 37°C, 5% CO 2 Incubate for 48 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Ic50 | aaaaa | aaaaa |
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com