Method for producing aluminum hydroxide from sodium aluminate solution
A technology of aluminum hydroxide and sodium aluminate, which is applied in the field of production of aluminum hydroxide, can solve the problems of long crystallization decomposition time, low material circulation efficiency, low decomposition rate, etc., achieve shortened reaction time, increase unit production capacity, and decomposition rate Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
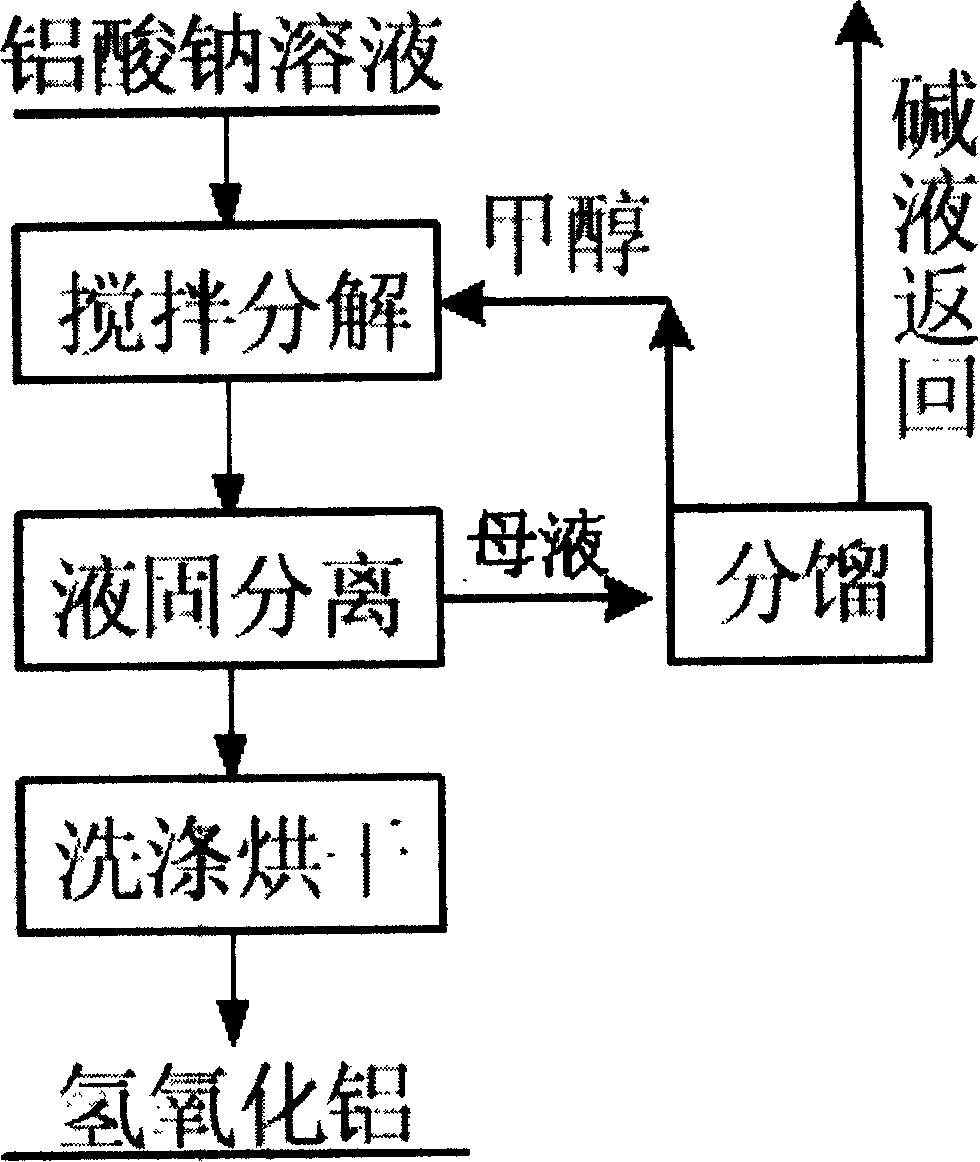
[0028] This embodiment includes the following steps:
[0029] 1) to Al 2 o 3 Concentration is 50g / L, in the sodium aluminate solution that caustic ratio is 1.4, add methanol, wherein, the volume ratio of two kinds of solutions is sodium aluminate solution: methanol=1: 0.6;
[0030] 2) Put the above-mentioned mixed solution in a reaction vessel kept at 30° C. for airtight stirring, and stop the reaction after half an hour;
[0031] 3) Liquid-solid separation of the reaction product in step 2) to obtain aluminum hydroxide solid phase and mother liquor; the content of sodium oxide in the mother liquor is 35g / L, and the caustic ratio is 13; the solid phase is washed in 50°C washing water for 3-5 times Dry at 60°C after slurry washing to obtain aluminum hydroxide product, and the decomposition rate of sodium aluminate in this example is 89%.
[0032] The scanning electron microscope of example 1 gained aluminum hydroxide is as figure 1 As shown, the particle size of the product...
Embodiment 2
[0034] In this example, Al in the sodium aluminate solution 2 o 3 The concentration is 100g / L, the caustic ratio is 1.4, the volume ratio of each solution in the initial mixed liquid sodium aluminate solution:methanol=1:1.5, the reaction temperature is 60°C, the reaction time is 8 hours, the washing water temperature is 90°C, and the drying temperature It is 110 DEG C, and all the other conditions and steps are the same as in Example 1. After the reaction, the concentration of sodium hydroxide in the mother liquor was 45g / L, the caustic ratio was 50, and the decomposition rate of alumina was 94%.
[0035] The mother liquor obtained above is subjected to vacuum fractionation at 65° C., and the obtained low boiling point component is methanol, which can be returned to step 1) and reused for preparing sodium aluminate solution.
Embodiment 3
[0037] Al in the sodium aluminate solution in the present embodiment 2 o 3 The concentration is 150g / L, the caustic ratio is 2.6, the mixed liquid is configured according to the volume ratio of sodium aluminate solution:methanol=1:3, the reaction time is 12 hours, the washing water temperature is 70°C, and the drying temperature is 80°C. The rest of the conditions and steps are the same Example 1. After the reaction, the concentration of sodium hydroxide in the mother liquor was 50 g / L, the caustic ratio was 13, and the decomposition rate of alumina was 80%.
[0038] The mother liquor obtained above is subjected to vacuum fractionation at 80° C., and the obtained low boiling point component is methanol, which can be returned to step 1) and reused for preparing sodium aluminate solution.
[0039] The scanning electron microscope of example 3 gained aluminum hydroxide is as figure 2 As shown, the particle size of the product is around 500nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com