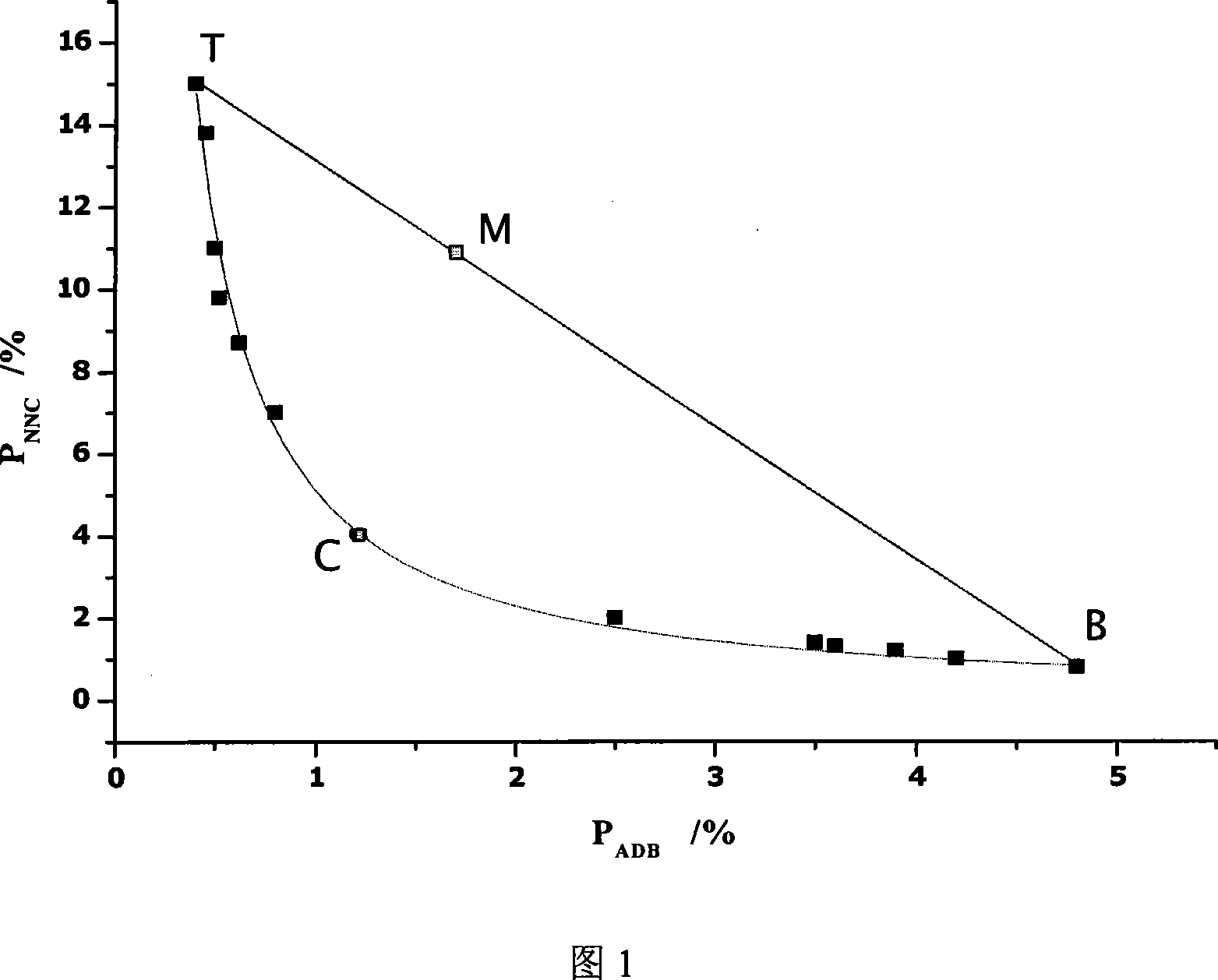
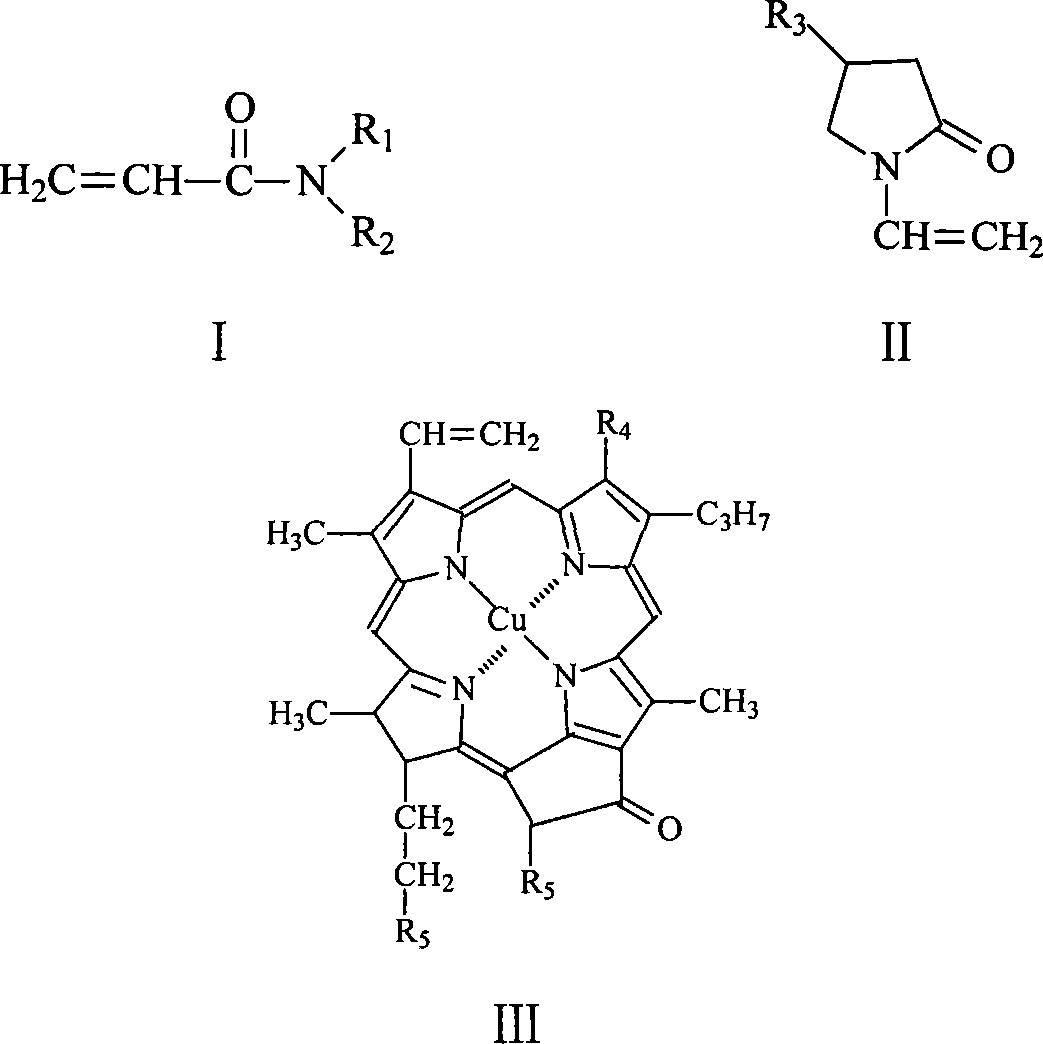
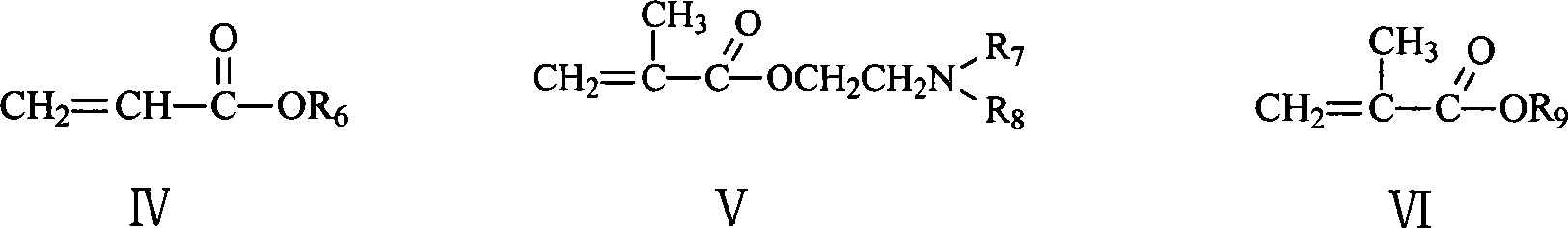Polymer capable of composing whole reused double water phase system
A polymer, phase-forming technology, applied in the polymer field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The preparation of the compound shown in formula V is based on methacryloyl chloride as a raw material, and the compound shown in formula VII is condensed with acryloyl chloride and formula VII using the prior art to obtain it (removing a part of HCl), and its synthetic route is as follows:
[0020]
[0021] In addition, the compounds represented by formula II, formula III, formula IV, formula VI and formula VII are all commercially available.
[0022] The method for preparing a pair of phase-forming polymers that can constitute a fully reusable two-phase aqueous system according to the present invention, its main steps are as follows:
[0023] ■Using benzene, anhydrous ether, tetrahydrofuran or ethanol as a solvent and azobisisobutyronitrile (AIBN) as an initiator, the molar ratio of the compound shown in formula I, the compound shown in formula II and the compound shown in formula III is 196 : 8: 1 into the solvent, in the presence of an inert gas, polymerize at 50...
Embodiment 1
[0028] Photosensitive Copolymer P NNC preparation:
[0029] Add 22.632g (0.2mol) isopropylacrylamide, 0.912g (0.0082mol) vinylpyrrolidone, 0.724g (0.001mol) sodium copper chlorophyllin in a stoppered Erlenmeyer flask, fully dissolve under stirring with 150ml benzene, 1.0 %molAIBN is used as an initiator, and nitrogen gas is filled for 10 to 15 minutes to fully remove oxygen. A stopper was used to seal the mouth of the Erlenmeyer flask, and the reaction was carried out on a water-bath shaker at 70° C. at 250 rpm for 24 hours. After the reaction was completed, the product was precipitated with n-hexane, and the precipitate was dried to constant weight to obtain the final product with a viscosity-average molecular weight of 7.1×10 3 . Because the sodium copper chlorophyllin monomer can absorb visible light, the maximum absorption wavelength of the polymer is determined to be 406nm.
Embodiment 2
[0031] pH sensitive copolymer P ADB preparation:
[0032]Add 33.33ml (0.42mol) acrylic acid, 26.67ml (0.15mol) 2-methylaminoethyl methacrylate, 3.33ml (0.021mol) tert-butyl methacrylate in a stoppered Erlenmeyer flask, the pH value in 200ml is 7.03 Fully dissolved in deionized water, 1.3% ammonium persulfate and sodium bisulfite (APS-NaHSO 3 ) composed of a composite redox system as an initiator, nitrogen and deoxygenation for 10 to 15 minutes. The reaction vessel was sealed, and the reaction was carried out at 55° C. and shaken in a water bath at 250 rpm for 24 hours. After the reaction was completed, the product was precipitated with petroleum ether, and the precipitate was dried to constant weight to obtain the final product. Determine its isoelectric point PI = 4.1. The polymer has a maximum UV absorption at 210 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com