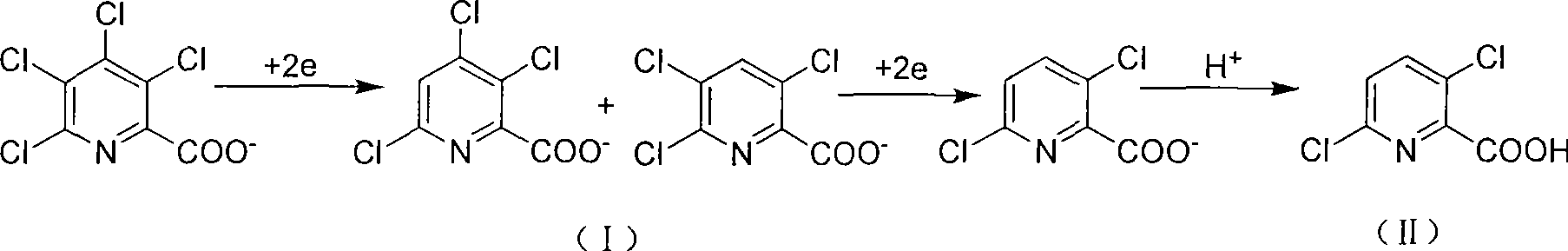
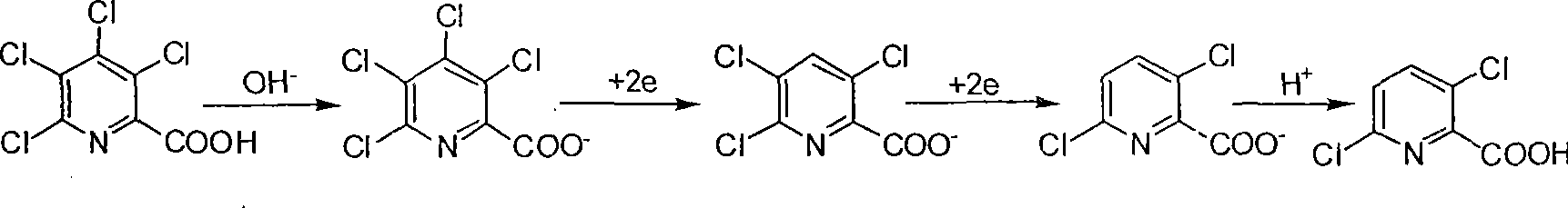
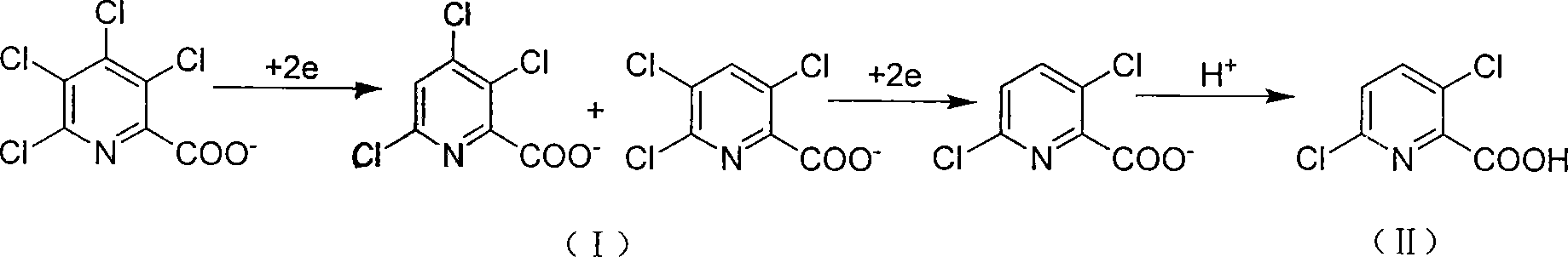
Electrolytic synthesis method for 3,6-dichloropicolinic acid
A technology of clopicolinic acid and tetraclopyridine, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems that the feeding speed cannot be too fast, the feeding time is long, and the reaction time is long, etc. Effects of treatment process, improvement of solubility performance, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 500ml beaker, dissolve 36.9g (0.128mol) and 20.5g (0.19mol) of sodium carbonate at 65°C in 270ml of distilled water with a content of 98% TCPANa (sodium tetraclopyridine), and install the electrodes under the same temperature conditions During the electrolysis reaction, 2.5ml 10mol / L NaOH (sodium hydroxide) solution was slowly added dropwise to maintain the alkaline range of the system at pH 9.5 to 11. After about 1.5 hours of reaction, the TCPANa content was lower than 1% (HPLC detection). Cool down to 25°C, and add 26.5ml of 10mol / L NaOH solution in batches to maintain the alkalinity of the reaction system to pH 13-13.5, control the cathode potential to -1.30v relative to the calomel electrode, and continue the reaction for 4 hours to complete. After cooling and filtering the electrolyte, adjust the pH of the electrolyte to 1.0 with concentrated hydrochloric acid, cool, crystallize, filter, and dry to obtain 21.6 g of white needle-like crystals of 3,6-dichloropico...
Embodiment 2
[0022] In a 500ml beaker, dissolve 98% TCPA Na (sodium tetraclopyridine) 45.3 (0.157mol) and 2.65g (0.025mol) of sodium carbonate and 270ml of distilled water at 65°C, install electrodes for electrolysis, batch 24.5g of sodium carbonate was added once, and it was finished within half an hour to maintain the system's alkaline range of pH 8.5 to 9.5. After reacting for about 2 hours, the content of TCPANa was lower than 1% (detected by HPLC). Lower the temperature to 30°C, add 33ml of 10mol / L NaOH solution in batches, maintain the alkalinity of the reaction system to pH 13-13.5, control the cathode potential to -1.30v relative to the calomel electrode, and continue the reaction for 4 hours to end. After the electrolyte is cooled and filtered, the pH of the electrolyte is adjusted to 1.0 with concentrated hydrochloric acid, then cooled, crystallized, filtered, and dried to obtain 26.5 g of white needle-like crystals of 3,6-dichloropicolinic acid. After the filtrate is concentrated...
Embodiment 3
[0024]In a 500ml beaker, dissolve 26.6g (0.1mol) and 26.5g (0.25mol) of sodium carbonate with a content of 98% TCPA (tetraclopicralic acid) in 270ml of distilled water at 65°C, and install electrodes for electrolysis. The NaOH solution of 2ml 10mol / L can be slowly dripped into the medium to maintain the alkalinity of pH 9.5~11 of the reaction system. After reacting for about 1.5 hours, the content of TCPANa is lower than 1% (HPLC detection). Cool down to 30°C, and add 20ml of 10mol / L NaOH solution in batches to maintain the alkalinity of the reaction system to pH 13-13.5, control the cathode potential to -1.30v relative to the calomel electrode, and continue the reaction for 4 hours to complete. After cooling and filtering the electrolyte, adjust the pH of the electrolyte to 1.0 with concentrated hydrochloric acid, cool, crystallize, filter, and dry to obtain 16.8 g of white needle-like crystals of 3,6-dichloropicolinic acid. After the filtrate is concentrated, extract with dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com