Anticoagulation agent and uses thereof
An anticoagulant, a technology for inhibiting coagulation, applied in the branch of hemostasis and in the field of hematology, which can solve the problem of wrong targeting of anticoagulants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
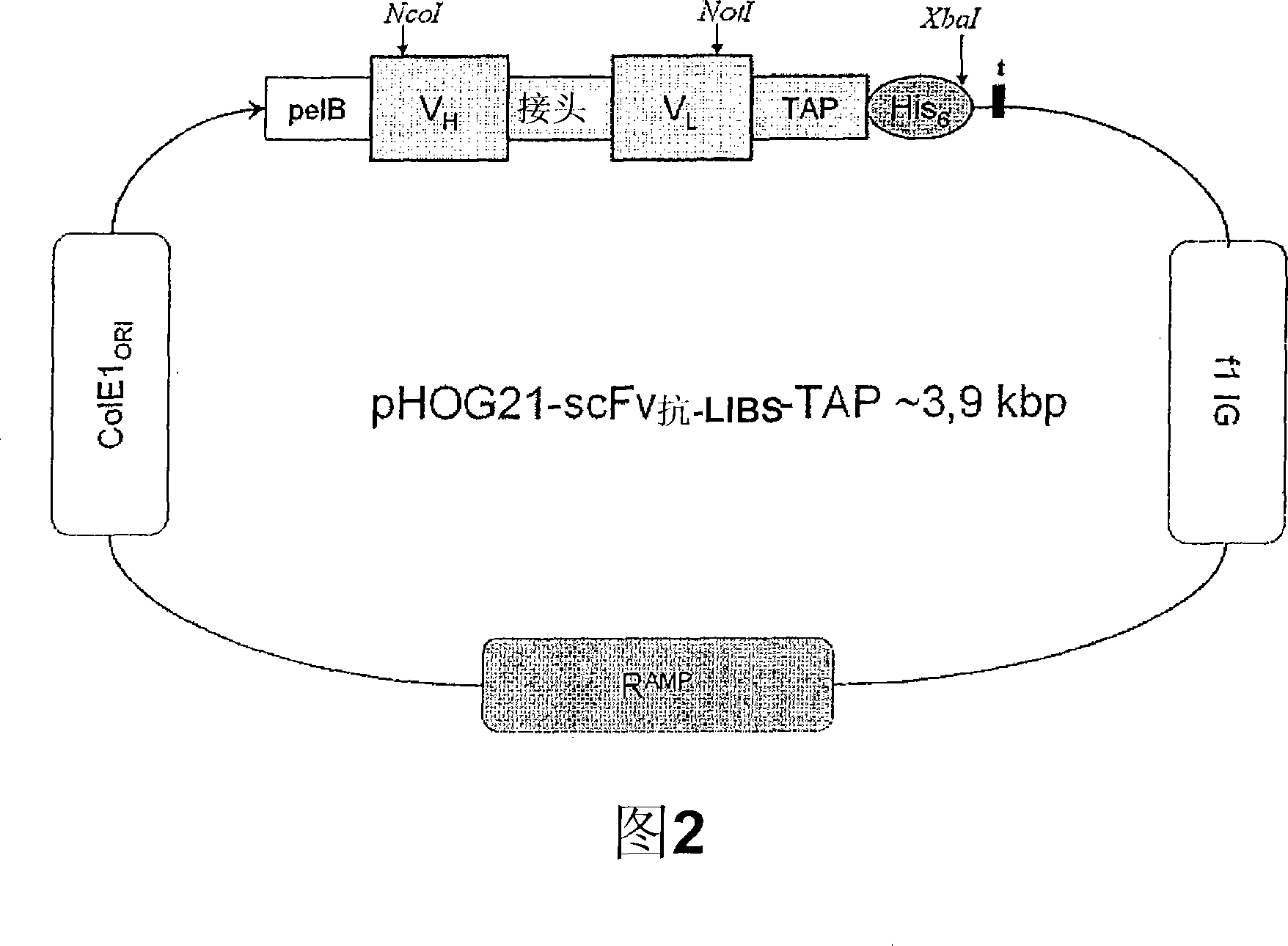
[0116] Example 1: Generation of single-chain antibody scFv 抗-LIBS and fusion construct scFv 抗-LIBS -TAP
[0117] Past literature has described the generation and functional characterization of hybridoma cell lines expressing monoclonal antibodies directed against LIBS epitopes on GPIIb / IIIa (Schwarz et al., JPET 2004;308:1002). Briefly, GPIIb / IIIa purified and eluted with RGD peptides was used as immunogen for producing hybridomas. Clones were screened with activated platelets and with immobilized GPIIb / IIIa saturated with RGD peptides. One of these clones, the monoclonal antibody (mAb) clone 145, was shown to bind to ADP-activated platelets as well as with RGD peptides (GRGDSP, BIOMOL Research Laboratories, Plymouth Meeting, PA), eptifibatide (Integrilin , Essex Pharma, Muenchen, Germany), tirofiban (tirofiban) (Aggrastat , MSD, Whitehouse Station, NJ) and abciximab (abciximab) (ReoPro , Eli Lilly & Co, Indianapolis, IN) increased binding of pre-incubated platelets...
Embodiment 2
[0119] Example 2: Expression and purification of scFv constructs in E. coli
[0120] Escherichia coli (TG1) cells were transformed with the above pHOG21 plasmid and single colonies from streaked agar plates were grown at 37°C in 500 mL flasks in LB medium containing 100 μg / mL ampicillin and 100 mM glucose. The culture was shaken at 200 rpm for approximately 4-6 hours until an OD (600 nm) of -0.8 was reached. Bacteria were pelleted by centrifugation at 5000 rpm for 10 min at 4°C, and resuspended with LB medium containing 100 μg / ml ampicillin and 0.4 M sucrose. IPTG was added to a final concentration of 0.25 mM to induce scFv production and incubated at room temperature (22-24° C.) at 200 rpm for 16-20 hours. For purification of soluble proteins from whole cell extracts, bacteria were harvested by centrifugation at 5000 rpm for 10 min at 4°C. Pellets of bacteria were resuspended in 5mL 1X BugBuster (Novagen, Madison, USA) solution / g bacterial mass, and incubated at room te...
Embodiment 3
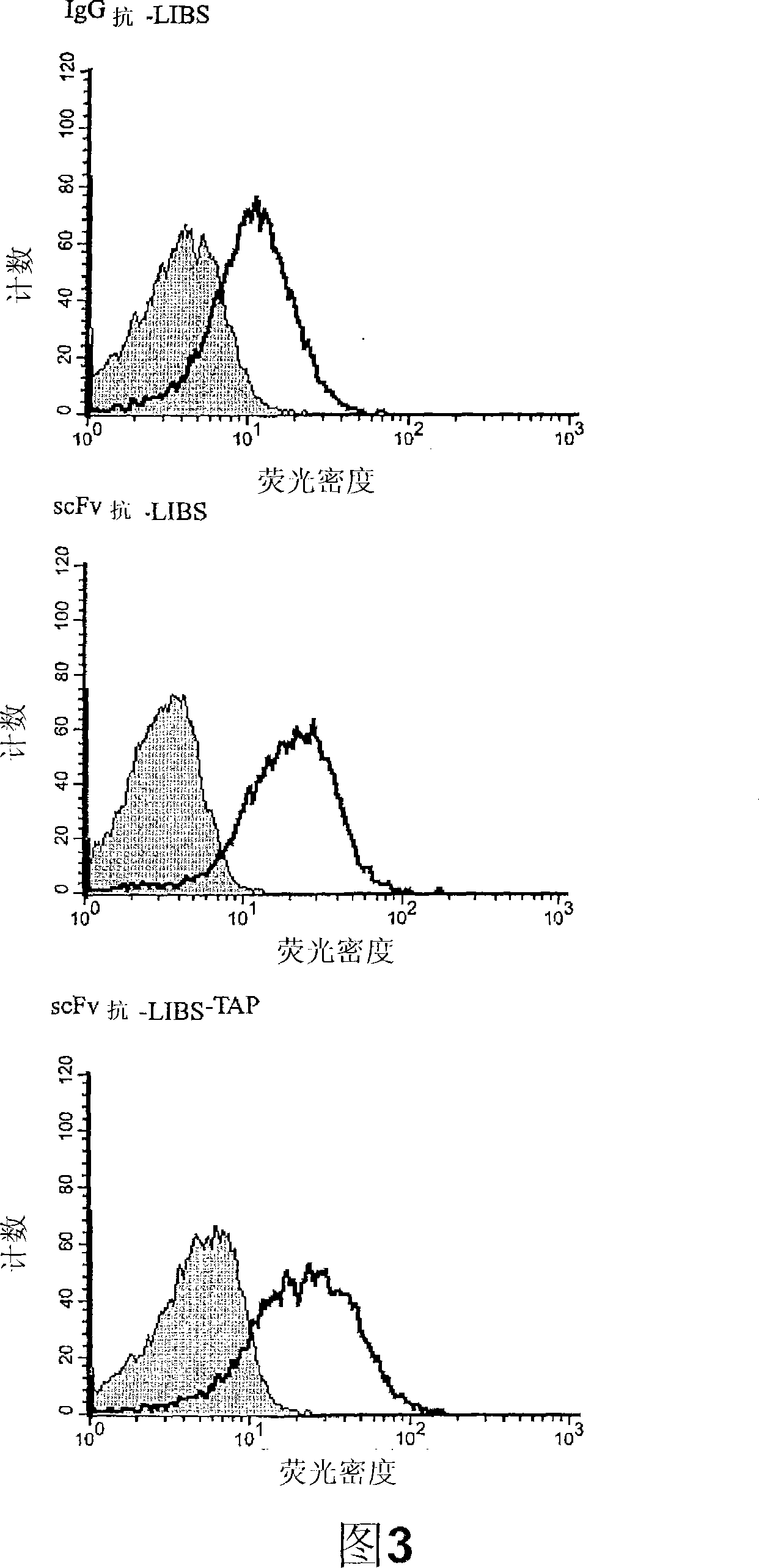
[0121] Example 3: In vitro functional properties of scFv anti-LIBS-TAP
[0122] blood products
[0123] Human blood was collected from healthy volunteers by venipuncture using a 21-gauge butterfly needle and anticoagulated with citrate. Platelet rich plasma was obtained by centrifugation at 100 xg for 10 min in a plastic tube at room temperature in a centrifuge (GS-6R centrifuge, Beckmann Coulter, Gladesville, NSW, Australia).
[0124] Mouse blood was collected from C57BL / 6 mice by intracardiac puncture using a 27-gauge needle and anticoagulated with unfractionated heparin (20 U / mL). In a volume of 50 μl, use 1 mL of modified Tyrode's buffer (150 mM NaCl, 2.5 mM KCl, 1.2 mM NaHCO 3 , 2mM MgCl 2 , 2mM CaCl 2 , 0.1% BSA, 0.1% glucose) were resuspended and centrifuged at 1300×g for 5 min. The supernatant was discarded, and the pellet was resuspended with 1 mL of modified Tyrode's buffer.
[0125] Flow Cytometry
[0126] Citrate-containing human whole blood was diluted 1 / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com